Responsible Innovation
#MoreThanMedicine
Responsible Innovation

Back innovation, Boost Access
Innovating responsibly also means making sure that medical innovation reaches patients in need without delays. Discover more about the industry's commitment to accelerating access to medicines.
Environmental sustainability
EFPIA and its members recognise the urgent need to address climate change and safeguard natural resources, given the profound impact on both human health and nature. We further acknowledge concerns of pharmaceuticals in the environment. It’s essential to move away from traditional methods and adopt innovative practices that reduce our environmental impact. We strive to go beyond compliance on the targets set within the various EU legislative requirements as part of the EU Green Deal initiatives under the Zero Pollution, Circular Economy and Climate Action plans. As leaders in the pharmaceutical sector, we are committed to taking decisive actions to reduce our environmental impacts across the value chain and contribute to building resilient and sustainable health system. We are leading the transition towards the decarbonisation in the pharmaceutical sector by setting ambitious science- based targets, investing in renewable energy, driving circularity, and collaborating with stakeholders.
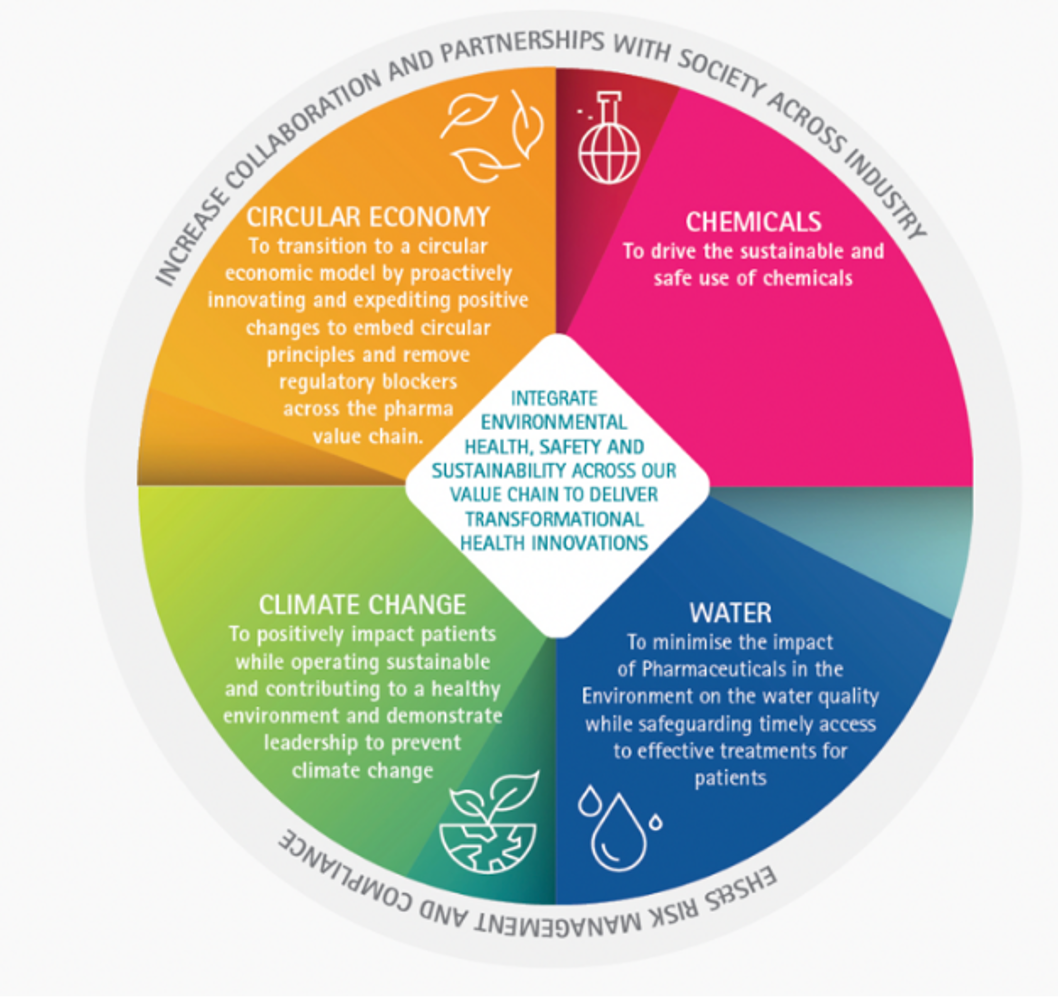
Climate action
Industry has put processes in place to build a healthier and more environmentally sustainable future. Pharmaceutical companies are driving an agile, innovative, evidence-based sustainability strategy enabling them to embrace evolutions in science, technology and society and to integrate sustainability across the entire value chain. This reflects industry’s drive to deliver quality, healthy and green outcomes while positively impacting on the lives of patients.
The industry encourages appropriate use of a risk-based approach to environmental challenges and undertakes initiatives to promote climate action by supporting:
- The principles in UN Global Compact on Climate Change [1]
- United Nations’ Sustainable Development Goal 13, aiming for urgent action to be taken to combat climate change and its impacts [2]
- The Paris Agreement adopted at the UN Climate conference in Paris (COP21) by supporting the long-term goal to hold the increase in global average temperatures well below 2°C and to pursue efforts to limit the increase to 1.5°C [3]
- The European Union’s ambition to be climate neutral by 2050 [4]
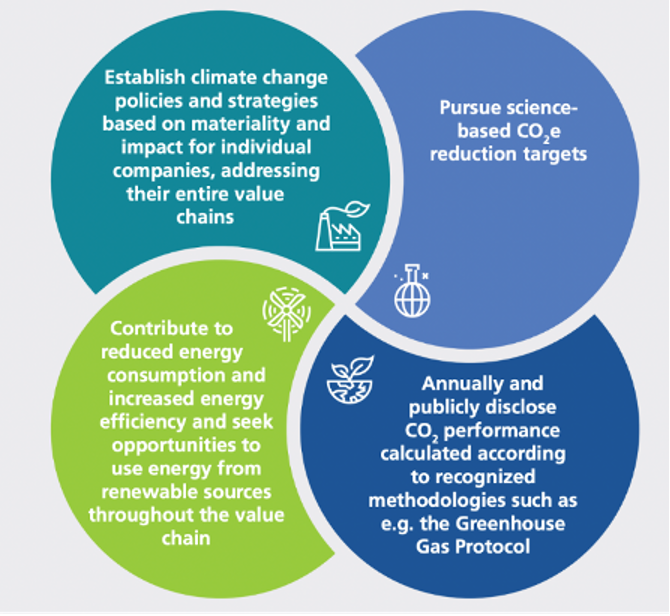
How industry is going green
EFPIA members are dedicated to making a positive impact on the lives of patients whilst operating in a sustainable manner. The EFPIA White Paper on the Circular Economy illustrates how companies have embraced reusable packaging and switched to specialised water treatment to recover valuable materials, and utilising waste and by-products for other purposes. The EFPIA White Paper on Climate Change provides examples of how manufacturers are working towards reducing Green House Gas emissions.
Case studies
A new solar park has been built through an agreement between Lundbeck and renewable energy company Better Energy . With this solar park, 100 percent of Lundbeck’s annual Danish electricity consumption is now matched by an equal amount of solar power. The solar power produced at this site feeds back into the national electrical grid, thus putting Lundbeck closer to net-zero carbon emissions globally. But the solar park is doing more than just greening Lundbeck; it’s a making significant contribution towards Denmark’s transition to a zero-carbon electricity grid.
Palladium is a precious metal with a critical role in the production of certain active pharmaceutical ingredients. The silver-white metal used in this process is rare, and J&J are always looking for ways to reduce wastage and maximize reuse . A partnership was set up with a specialized Flemish company focused on the circular economy who have a one-stop-shop concept that enables J&J to recycle the precious metal much faster and more efficiently. That means J&J can cut down CO2 emissions related to mining and refining of virgin Palladium, reduce logistics and eliminate additional processing costs.
The PSCI is a membership group of companies in the pharmaceutical and healthcare sector that commit to working together towards a common vision of excellence in our supply chains, focusing on ethics, human rights and labour, health and safety, environment and management systems . The foundational commitment of all members is to the PSCI Principles which are integrated into companies’ own supplier codes. In 2021 membership of the group grew, engagement of suppliers has increased and there are promising signs of this having a positive impact on supplier practices.
UCB aims to package medicines in the most environmentally sustainable way possible whilst ensuring compliance with regulations, on-pack labelling requirements, measures to combat counterfeit drugs, the need to implement practical features for patients, and enhancing therapeutic adherence. The latest in innovative packaging design incorporates innovative features focusing on pack size, recyclability, and sustainability to significantly reduce the company’s ecological footprint. As a result, UCB has reduced packaging waste by 15% in weight, improved storage efficacy (pack size reduced by 13%), and improved recyclability (plastic content is reduced by 85%). The UCB team has already been recognized through 7 eco-design PHARMAPACK Awards in the health product category –assessed by a professional jury made up of experts from leading research institutes and universities, industry and the trade press.
Chiesi launched the Take Action for Inhaler Recycling pilot scheme in a region of the UK in January 2021 . The initiative enables inhaler users to recycle their empty, unwanted or out of date inhalers safely and effectively. Inhalers are sent through the postal system, directly to a waste management company. Through the scheme, the aluminium canisters are crushed and recycled. The plastic components are recycled into the plastic supply chain and any remaining propellant gas is extracted and reused in the refrigeration and air conditioning industry. As of April 2022, patients have returned 6,491 envelopes, containing 24,469 inhalers and 144 tonnes of CO2 have been captured.
Pharmaceuticals can end up in the environment in many ways, primarily by excretion through the body. However, when unused medicines are not disposed of in the right way, such as flushing them down the toilet or pouring them down the sink, they can have an unforeseen impact on the environment. #Medsdisposal is a joint initiative between EFPIA, European healthcare, industry and student organisations to raise awareness on how to dispose of unused or expired medicines appropriately in Europe [5]. The initiative provides information for the public on how to dispose of their medicines in different European countries.
Collaborative approaches allow us to increase our mutual knowledge and understanding on how to proactively address any potential risks imposed by the presence of pharmaceuticals in the environment (PiE). To this end, EFPIA, AESGP and Medicines for Europe have developed the Eco-Pharmaco-Stewardship (EPS) framework that applies the widely accepted principles of product stewardship and is implemented across the industry and with broader stakeholders in the healthcare and environmental sector.[6]
The Energize programme was launched in 2021 as part of COP 26. It is a collaboration between 10 global pharmaceutical companies. It is a first-of-its-kind supplier program to advance climate action to support pharma supply chains to buy renewable energy. This gives suppliers who may not otherwise be able to participate in the market the scale, tools, and support to access utility-scale power purchase agreements – allowing them to reduce emissions.
Novo Nordisk produces more than 600 million injection pens globally per year equal to 20,000 tons of plastic. Today, used pens end up as waste in landfills, incineration plants or in nature. Solving the end-of-life product challenge is a key component of Novo Nordisk’ approach to contributing to a thriving circular economy. To address plastic waste and disposable devices, Novo Nordisk has introduced a TakeBack programme in Denmark with pilots launching in the U.K. and Brazil. Returned devices are broken into different components and given new life via a range of upcycling initiatives. The aim is to create an industry-wide TakeBack programme to facilitate a new and more sustainable way of handling medical device waste across all therapy areas.
The AMR Industry Alliance has launched a key policy designed to reduce the impact of microbial products manufacturing: Antibiotic Manufacturing Standard (AMS): Minimizing risk of developing antibiotic resistance and aquatic ecotoxicity in the environment resulting from the manufacturing of human antibiotics. The AMS aims to reduce the risk from antibiotic manufacturing emissions to surface water and the broader environment through the control of antibiotic residue in wastewater and solid waste. It requires an environmental management system and risk-based approach to assessing and controlling antibiotic manufacturing waste streams and adherence to the Alliance’s published Predicted No-Effect Concentrations (PNEC). [7]
Driving high standards in animal welfare and embracing the 3Rs
Animal models have played an important role in the development of medicines and vaccines. However, industry is striving to move away from research and testing involving animals where possible and is investing in developing alternatives for many of the processes where animals are still required. EFPIA and its members are committed to the science-based phase-in of methods to replace the use of animals for scientific purposes and the deletion of animal tests which are obsolete or redundant.
Since the adoption of the EU legislation governing animal use, EFPIA and its members have been publishing reports to visibly highlight industry actions on putting animal welfare principles and the 3Rs (replace, refine, reduce) into action [8]. This illustrates a continued drive to reduce the number of animals used, refine experiments to minimise the impact on animals, and replace animal experiments wherever possible with alternatives.
EFPIA members are aiming to lead progress by engaging in a wide range of practical activities to help drive the development, uptake and promotion of non-animal technologies (NATs) and new approach methodologies (NAMs) so that these can be phased-in as soon as it is scientifically possible to do so, as well as working on improving animal welfare.

Replace
Many animal experiments can be replaced with methods, strategies or approaches which do not involve the use of live animals.
- Accelerating Global Deletion of the Abnormal Toxicity Test (ATT)
The ATT test is currently required but results in the unnecessary use of animals without a scientific rationale. EFPIA is working with the Animal Free Safety Assessment Collaboration (AFSA) and Humane Society International (HSI), in collaboration with the International Alliance of Biological Standardization (IABS) to move towards removing the requirement to use this test. - Replacement method for environmental risk assessment for antibiotics
The availability of effective antibiotics is critical for patient health and the long-term sustainability of the healthcare system. For the regulatory approval of antibiotics an environmental risk assessment (ERA) is required that includes fish testing at the highest severity. Industry and academic researchers have shown that cyanobacteria can be used to assess environmental toxicity of antibiotics [9]. This led to a change in European Medicines Agency (EMA) legislation, removing the requirement for regulatory fish testing. - Replacement of animal use with human and artificial skin
Human skin from cosmetic surgery and highly standardized artificial skin can eliminate the use of animal testing and reduce variability of animal models, while providing an appropriate method to support ongoing quality assessment. Innovative solutions like mounting skin on warm liquid reservoirs and stretching the skin have also been developed to simulate intact, live skin.
Reduce
There are several opportunities to use fewer animals. The application of machine learning and artificial intelligence through in silico modelling are ever-expanding in the pharmaceutical industry and allows for a reduction in animal studies while ensuring products are robustly tested. Furthermore, reduction is possible through improve experimental design of experiments, re-use, combining animal experiments with in vitro models, and by using a single species.
Refine
Experiments can be refined to minimise the pain, suffering and distress experienced by the animal and enhance its well-being. For example, improved housing and care, consideration of routine handling for husbandry and experimental procedures; introduction of trainings; use of non-invasive methods for sampling or the use of digital technology to monitor animals.
Improving animal and human welfare
EFPIA and its members have been working to promote a good culture of care across research institutions and companies in Europe. They have developed resources to raise awareness around Culture of Care, including a framework [10] to help organisations identify gaps or potential areas for improvement and a pamphlet [11].
Further progress can be achieved by improving the monitoring of mouse welfare. The NC3Rs Crack It Challenge competition funds collaborations between industry, academics and SMEs to solve business and scientific challenges which will deliver 3Rs benefits. For example, mouse facial expression and body condition scoring are currently used as welfare assessment tools, but they have limitations. The NC3Rs challenge funded the development of Mouse Mapp. This uses artificial intelligence and machine learning to automatically detect changes in facial expression and/or body condition, to improve the monitoring of mouse welfare [12].
Collaborative research
Partnerships play a key role in advancing the 3Rs and raising standards across the industry. The European Partnership for Alternative Approaches to Animal testing is a cross-sectorial and multidisciplinary partnership between five European Commission services, 37 companies and eight industry sector associations. EFPIA and a number of its member compounds are founding members of the EPAA and strong supporters of its mission to promote the 3Rs in regulatory testing, and facilitate the development and regulatory acceptance of alternative testing strategies.
Collaboration between industry, academia, regulators and others has also advanced 3Rs research. The Innovative Medicines Initiative (IMI) has made a significant contribution to animal welfare through public-private partnership [13].EFPIA Code
Industry understands the importance of working in an open and transparent way with health professionals, healthcare organisations, patients and the public in accordance with legislation and in an ethical framework. EFPIA Ethical Principles are included in the EFPIA Code of Practice, which is continually reviewed and adapted over time to reflect societal expectations for ethical business. 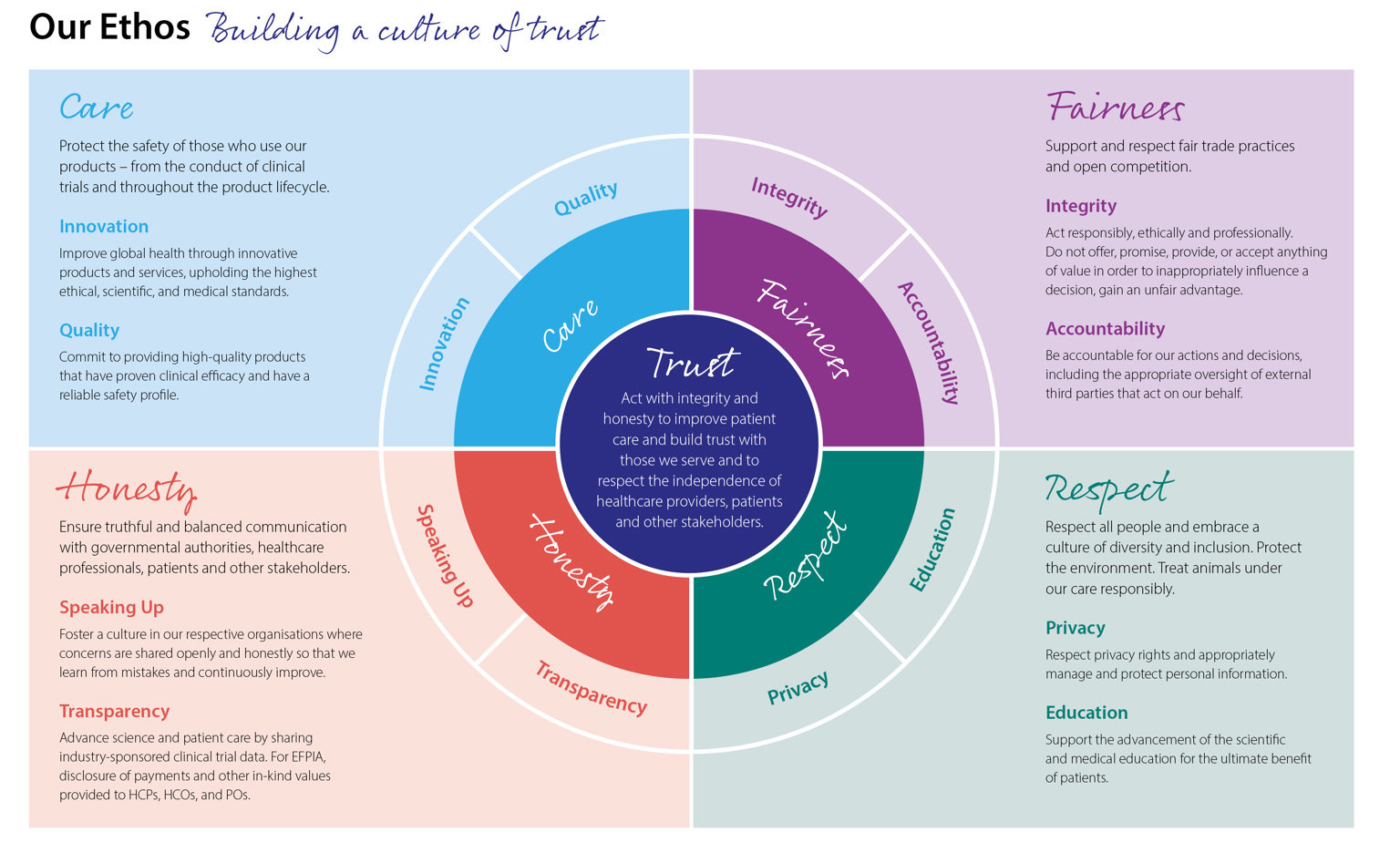
Driving high ethical standards
The EFPIA Code governs pharmaceutical industry interactions with stakeholders including healthcare professionals, healthcare organisations and patient organisations across Europe. Compliance with the Code is documented in an annual report from EFPIA which covers activities in different countries.
The 2021 report showed that industry continued implementing and applying the highest ethical standards as well as applicable laws and regulations such as competition, data protection, anti-bribery and anti-corruption legislation. Guidance was issued to account for the impact of COVID on companies and the shift to virtual meetings and this was supported through a joint guidance from EFPIA, IFPMA and PhRMA.
The EFPIA Code is transposed into Codes in each country. In addition to having a local Code, countries carry out additional activities to support implementation of their Code and ethical interaction. Some examples are shown here.
-
France: Leem's charter for relations with patients and their representatives sets out the principles that underpin working relationships and collaborations [14].
-
Belgium: Pharma.be has developed infographics on ethical collaborations between the health sector on pharmaceutical companies [15][16].
-
UK: The ABPI has produced a sourcebook to support pharmaceutical companies in working successfully and collaboratively with patients and patient organisations [17].
References
[1] Climate Change | UN Global Compact
[2] Goal 13 | Department of Economic and Social Affairs (un.org)
[3] The Paris Agreement | United Nations
[4] 2050 long-term strategy (europa.eu)
[5] http://medsdisposal.eu/about-us/
[6] https://www.efpia.eu/media/636524/efpia-eps-brochure_care-for-people-our-environment.pdf
[7] AMR Industry Alliance Launches Antibiotic Manufacturing Standard to Help Mitigate the Impacts of Antimicrobial Resistance - AMR Industry Alliance
[8] putting-animal-welfare-principles-and-3rs-into-action.pdf (efpia.eu)
[9] Variability in cyanobacteria sensitivity to antibiotics and implications for environmental risk assessment - PubMed (nih.gov)
[10] https://journals.sagepub.com/doi/full/10.1177/0023677219887998?journalCode=lana&
[11] https://www.efpia.eu/media/602714/culture-of-care-2021-leaflet.pdf
[12] Mouse MApp | Innovation Platform (nc3rs.org.uk)
[13] https://www.imi.europa.eu/news-events/newsroom/focus-replace-reduce-refine
[14] /Charte du Leem vis-à-vis des relations avec les patients et leurs représentants | Leem">Charte du Leem vis-à-vis des relations avec les patients et leurs représentants | Leem
[15] https://pharma.be/sites/default/files/2021-08/health_ethics_fr.pdf
[16] health_ethics_nl.pdf (pharma.be)
[17] Working with patients and patient organisations - a sourcebook for industry (abpi.org.uk)
