Why we need an open infrastructure for proactive patient engagement throughout the lifecycle of a product (Guest Blog)
22.10.15
Patient engagement today is a fragmented environment, and there is no organised information repository to share approaches, practices, standards, and regulatory provisions, nor any guidance on how to use the few tools available.
Good examples of patient engagement practices are emerging but, to date, stakeholder sharing of practices is rare, and existing, expert, consensus-based guidance on how to undertake patient engagement effectively is limited2,3. Likewise, it remains unclear how to assess whether a particular approach to patient engagement at different time points in the therapeutic innovation process will yield meaningful results for all parties involved. From a patient community perspective, there is also a lack of clarity about how they can initiate this patient engagement or how their input will be organised and used to inform the process, as well as how the perspective of their experience will be included4.
From an academic research and drug developer perspective, there is little expert guidance and lack of consensus to determine: who should be involved; how and when; what role the patient community should have; and at what points in the development process the patient community should be engaged, to inform an approach effectively. It is clear that a failure to gain patient insights at the right time can lead to misguided priorities and costly, late-stage failures.
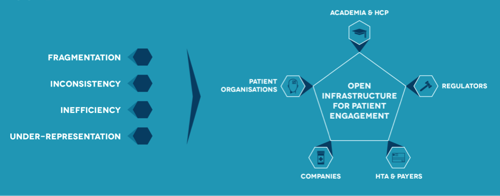
It is therefore critical to develop standard approaches and guidance that are shared and embraced by all stakeholders, and made available and accessible through an easy-to-use tool. All parties involved in medicines development recognise the need to establish a European platform that will provide a single place for capturing and sharing practices, and providing assistance for patient engagement for all diseases, adult and paediatric.
In order to ensure a genuine patient-centred model, all stakeholders operating in the field should engage in multi-disciplinary cooperation: industry (pharmaceutical companies, SMEs, biotech, device companies and start-ups); patients and patient groups/organisations; healthcare professionals and their associations; academic research groups; regulatory bodies (EMA, European network of Head of Agencies, national regulatory agencies); Health Technology Assessment agencies, the European HTA Network [EUnetHTA] and national HTA bodies); payers and purchasers of medicines and health products; and ethics committees.
In response to this rapid evolution, the patients’ contribution and responsibility is to provide high quality cross-disease, or disease-specific, knowledge and expertise to help guide and refine the research, development and assessment processes. It is widely acknowledged today by the different stakeholders involved in medicines development, that innovative approaches, creative thinking and robust, trusted and ethical practices are needed to orientate research and medicines development towards better patient health outcomes. Therefore, we need a set of comprehensive resources and services, organised in an open infrastructure that will enable information and know-how sharing, providing non-binding guidance and timely user-friendly material, which will enhance mutual trust among the various stakeholders in the patient engagement process.
This would address the current fragmentation, inconsistency, inefficiency, and under-representation that taints patient engagement throughout the lifetime of a medicine, by delivering a set of unique and sustainable solutions for all stakeholders involved. It would also facilitate the structured engagement of patients with academia and industry sponsors – and with public health authorities’ assessors – throughout the product lifecycle . These resources would impact medicines research and development process in two ways: medicines developed with patient input will deliver more value to patients and society as a whole; and the development process will become more efficient (e.g. faster recruitment in clinical trials, less drop out, better endpoints and endpoint measurement procedures, more patients reporting on adverse events or outcomes).
1 Smits E, Boon W. The role of users in innovation in the pharmaceutical industry. Drug Discovery Today 2008; 13 (7/8): 353-9.
2 Houyez F. Active involvement of patients in drug research, evaluation and commercialisation: European perspective. The Journal of Ambulatory Care Management 2004: 27 (2), 139-145.
3 Hoos A, Anderson J, Boutin M et al. Partnering with patients in the development and lifecycle of medicines: A call for action. Therapeutic Innovation and Regulatory Science 2015, 1-11.
4 Parsons S, Starling B, Tham SG et al. Involving patient representatives in the medicines development process – current capacity and expertise, opportunities and challenges. 2015 In preparation.
