IMI JU and EFPIA – New Drugs for Bad Bugs programme (ND4BB) (Guest blog)
17.11.15
- Scientific challenges to understanding and accessing drug targets, and overcoming hurdles to developing clinical candidates
- Development challenges to undertaking clinical trials to provide efficacy and safety data on new therapies for the prevention and treatment of serious bacterial infections
- The challenge of developing business models to both reward appropriately antibiotic R&D and to ensure responsible use of antibiotics
There are now seven projects launched within ND4BB, covering all the key areas of antibacterial research and development:
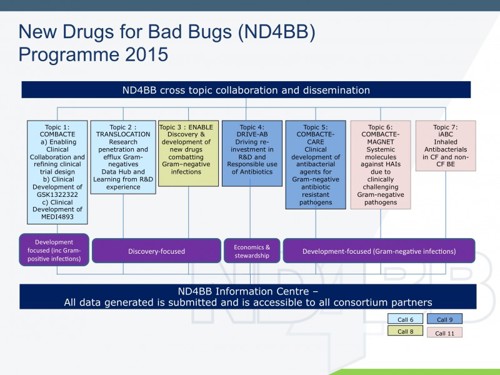
These projects are unique collaborations in which the academic and industry partners work together on a shared goal of addressing antibiotic resistance. I am delighted to say that clinical trials within COMBACTE actively are recruiting patients to assess novel preventative therapies and extensive observational clinical research is underway to understand the clinical management of difficult-to-treat and resistant bacteria. Active preparations are in place within COMBACTE-CARE and COMBACTE-MAGNET to start therapeutic trials, investigating β-lactam/β-lactam inhibitor combinations to treat patients with carbapenem-resistant infections. TRANSLOCATION is developing guidelines for designing and developing new drugs to tackle antibiotic resistance and is creating an information centre for pre-existing and ongoing antibacterial research data. ENABLE is supporting new discoveries and compounds coming from universities and small- to medium enterprises, with new programmes being evaluated and joining the consortium and benefiting from industry’s experience. iABC has been launched recently with the aim of developing novel inhaled antibiotics to treat chronic lung infection, the main cause of disease and mortality in patients with cystic fibrosis and bronchiectasis. DRIVE-AB is a unique project aim at developing and exploring alternative economic models to incentivise R&D, while encouraging the responsible use of antibiotics. After only 1 year, the project is already an influential contributor to the discussions ongoing at national governmental and international levels.
The concept of collaboration and cost-sharing has been recognised recently by both the UK government-commissioned ‘Review on Antimicrobial Resistance’, led by Jim O’Neil, and the US government’s Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria. Therefore, the contribution made by the IMI JU, with EFPIA supporting collaborative funding, into the R&D of antibiotics is leading the way and represents a substantial long-term investment in European antibacterial R&D capability and bacterial disease prevention and treatment.
About the author
Dr Seamus O’Brien
Executive Clinical Director – AstraZeneca Antibiotics Business Unit
The antibiotics business unit is dedicated to the development of treatment options for serious and resistant bacterial infections.
Seamus is the medical science lead for the development of a β-lactam/β-lactamase inhibitor combination for the treatment of multi drug resistant Gram-negative bacterial infections.
He is responsible with colleagues from GSK for setting up COMBACTE, the first clinical development project within New Drugs for Bad Bugs supported by the IMI Joint undertaking with EFPIA. Seamus is currently Deputy Coordinator for COMBACTE and Coordinator for COMBACTE-CARE, a recently launched project with the aim of understanding how patients with carbapenem resistant Enterobacteriaceae (CRE) infections are managed with a view to supporting the development of treatment options.
He is also Co-Chair of the ND4BB Coordination team.
