From concept to approval –An IMI project is born
20.07.16
Innovation is the driver of the European economy and there is no single area where this is more evident than in healthcare. The Innovative Medicines Initiative, one of the Horizon 2020 research funding tools and the world’s largest public-private partnership (PPP) in the life sciences sector, acts both as generator and accelerator of innovation.
The question is how does this all work? What is the process involved in bringing an idea from concept to actual project? It is already handily described by a simple, yet informative infographic that can be found below. Nevertheless, walking through it in words may help to shed further light on this issue and encourage potential partners to sign up.
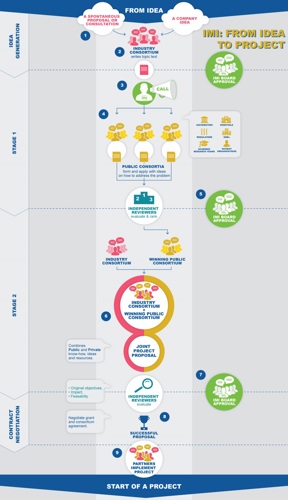
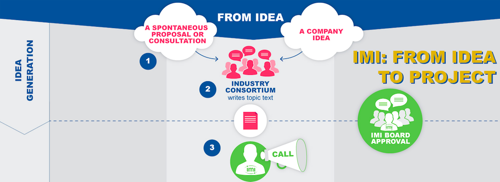
The quest for an IMI proposal begins with an idea. This can be generated by a company, which may or may not already have other companies in mind for a possible collaboration. Alternatively the idea may simply be a spontaneous spark submitted to EFPIA from another source.
The idea takes the form of a question or problem that requires resolution. This is effectively a problem that is common to several companies, and one that cannot be solved simply by companies joining forces. Therefore input from other parties – academia, SMEs, regulators, patients, etc. – is needed. This problem statement – and related questions that need to be addressed jointly by public and private partners – is written up as a topic.
Based on the text of this topic – and presuming the topic is robust and viable, i.e. it contains a sufficient level of investment from various companies and other investors (see our infographic)– IMI will then issue a “Call”, the precise text of which is subject to approval by the IMI Board. The Call, a type of tender, invites consortia populated by public bodies to submit proposals for projects. Essentially, these are all bodies that may benefit from public funding and industry plays no part in their formation or selection.
The proposals from public consortia are then subject to scrutiny by independent reviewers. The panel of independent experts in the topic and the review process are organised by the IMI office. Based on the reviewers’ analyses and ranking recommendations, it falls to the IMI Board, once again, to approve the successful applicants.
This is when real, universal collaboration kicks in, as the winning public consortium merges with an industry consortium (representing the private element of the PPP). Together they create and submit a joint proposal to IMI and the independent reviewers.
There is, though, no automatic rubber stamp for this proposal. The reviewers once again analyse the proposal and recommend the approval or rejection of it. On this basis, the IMI Board will approve funding for successful joint projects and the public private consortium will negotiate the contractual terms that will move the project forward to implementation.
An IMI project is born!