EFPIA/CPIA Joint China/EU Pharmaceutical Industry Forum Showcases Progress in Environmental, Regulatory and Innovation Issues
19.04.18
While delegates to the fourth EFPIA/CPIA Joint China/EU Pharmaceutical Industry Forum were granted an opportunity to explore the incredible progress that China has made in the field of pharmaceutical regulation, they were also given a taste of the exciting innovation to come.
The proceedings opened with an in-depth survey of the state-of-play in the Chinese pharmaceutical market, offered by the China Pharmaceutical Industry Association (CPIA). This highlighted the growth of the industry and its commitment to innovation with a view to full human health coverage in the domestic market and a renewed focus on exports.
This paved the way for me to explain how this progress in industry development has been matched by advances in the regulatory sector, under the auspices of the International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). By setting common standards in science and ethics, ICH has enjoyed significant success in ensuring that clinical trials conducted in one ICH region can be utilised in other ICH regions. Its development of harmonised regulatory guidelines is bringing the world closer together.
China is already making great strides in this arena, Mr Liu Xiaohan, Special Guest Vice President, CPIA and Director-General of the Hebei Pharmaceutical Association, explained, with the CFDA having translated all ICH guidelines into Chinese several years ago. In recent years, China has updated its legislation and guidelines in order to implement the basic ICH guidelines and work is also ongoing on the implementation of additional ICH guidelines.
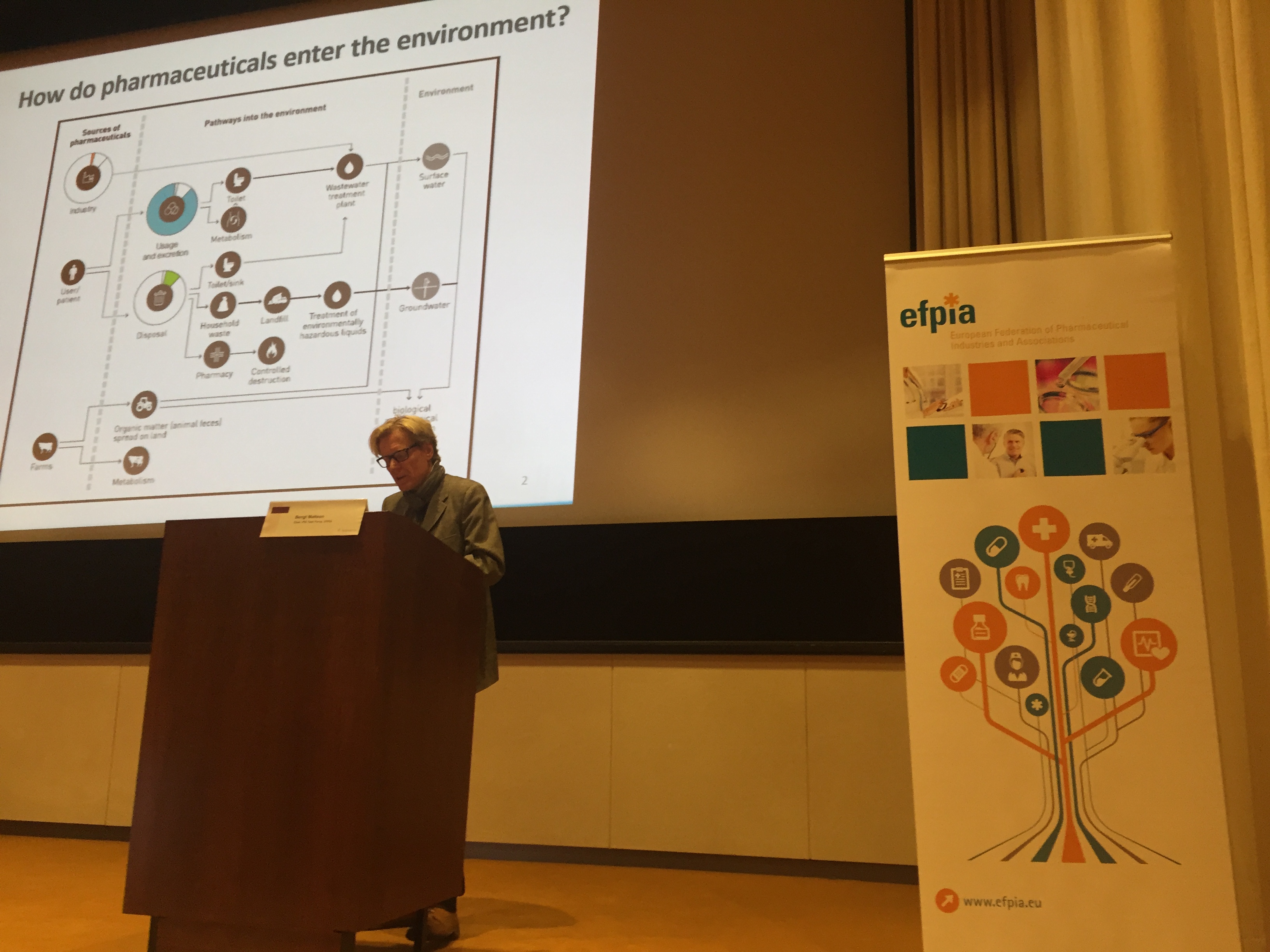
Industry growth in all ICH regions has also been accompanied by increased responsibility for the environment. Bengt Mattson clarified the European industry view on the European Commission’s proposals for pharmaceuticals in the environment (PIE). His view that that the future Commission PIE strategy could influence the rest of the world via work being undertaken in United Nations was of particular interest.
The proceeding presentation by Dr. Hao Hang was a true eye-opener, opening on the subject of continuous manufacturing and focusing specifically on flow chemistry technology. A highly-technical presentation, it showed how this process could save costs and reduce environmental pollution. Uptake in the pharma sector is not yet as speedy as it might be, because an upfront investment is necessary. Nevertheless the continuous manufacturing angle is especially topical as it is currently being discussed within ICH.
The focus of the forum now shifted to compliance, with delegates gaining insight into efforts undertaken in China to set up a self-regulation system. This was mirrored by a presentation from Jürg Granwehr, head of pharma at ScienceIndustries, the Swiss business association for the chemical, pharmaceutical and biotech industries. He explained how Swiss self-regulation was so well-functioning that arbitration rather than the threat of financial sanction was the norm in cases of non compliance.
Patrizia Tosetti from the European Commission gave an extensive overview of the identification and authentication of medicines in Europe. She focused on the Falsified Medicines Directive, which will reach final implementation on 9 February 2019. Andreas Walter, General Manager of the European Medicines Verification Office, explained the role of European Medicines Verification System, based on the central European hub. He offered some cause for concern, however, when he highlighted the fact that of over 2000 stakeholders who were supposed to have onboarded to the system by uploading their data, only some 70 had already done so at this stage.
This informative and compelling set of presentations was rounded off with a panel discussion on the entire range of topics, chaired by Faraz Kermani. It featured further in-depth discussion and also significant participation from the floor.
To conclude, this year’s forum workshop was a resounding success, building on the obvious progress of previous years. It can only be hoped that this success is reflected in next year’s forum, which will take place in China.