ISENTRESS: integrase strand transfer inhibitor active against HIV type 1
With over 7000 medicines in development, new treatments will continue to change patients’ lives; slowing disease progression, avoiding illness and reducing overall costs for healthcare systems. But developing a new medicines is a long, complex and risky process with no guarantees of success. Over the coming weeks we look at number of new medicines and role pharmaceutical incentives or IP has played in their development.
Of almost 37 million people worldwide infected with the human immunodeficiency virus (HIV), around 1.8 million are children up to 15 years old[1]. HIV has two strains; HIV-1 being the most widespread and severe and this is commonly referred to as HIV.
This virus attacks the human immune system, making it harder for the body to fight infections and disease. If left untreated, it can lead to acquired immunodeficiency syndrome (AIDS) and, consequently, death. The disease is more severe in children, because the virus is mostly transmitted from the infected mother during pregnancy, childbirth, or breastfeeding, when the child’s immune system is still developing. While the mortality rate of untreated HIV-1 infection in infants is high, early diagnosis and treatment of infected infants has reduced both mortality and disease progression by approximately 75%[2].
Although there is no cure for HIV, proper treatment is essential in order to keep the virus suppressed, thereby helping people living with HIV and – importantly –preventing its spread. However, the greatest burden of the infection is in low- and lower-middle income countries, especially sub-Saharan Africa, where there are fewer commercial incentives for the development of pediatric, optimised combination formulations.
Thus, infants and children either do not have access to treatment at all, or they have to adapt to medicines formulated for adults, which are not appropriate for paediatric use (unappealing taste, harder to administer, etc.). This leads to lower rates of treatment adherence in the youth population. Neonates in particular (whether HIV-1 exposed or infected) have very few antiretroviral treatment options, and represent an important unmet medical need.
Merck Sharp & Dohme (MSD) has been working for more than 30 years on addressing the global impact of HIV/AIDS. At the forefront of these efforts was the introduction 10 years ago, of the first agent of a novel class of HIV therapy, ISENTRESS® (raltegravir). It prevents HIV-1 growth by inhibiting integrase, which is a key enzyme required in the HIV replication process. When the integrase enzyme is blocked, the virus can no longer reproduce properly. In 2008, this outstanding invention was awarded the most prestigious international award for pharmaceutical research and development, the Prix Galien, in the “Best Pharmaceutical Product” category.
ISENTRESS® is now indicated in combination with other antiretroviral agents for the treatment of HIV infection, ensuring a greater likelihood of treatment response in patients four weeks of age and older. The regulatory process to extend the use of Isentress to neonates is currently ongoing.
New pharmaceutical formulations and paediatric clinical trials
Besides the film-coated tablets approved for administration in adults and in children of at least 25kg in weight, the approved Paediatric Investigation Plan (PIP) in the EU included studies for developing two additional child-friendly formulations: chewable tablets (evaluated in patients 2 to 12 years of age) and granules for oral suspension (evaluated in patients 4 weeks to 2 years of age). There were two clinical studies in children, including HIV-infected subjects, including adolescents (18 years of age) to infants 4 weeks of age, as well as HIV-exposed newborns (those born to HIV-1 infected mothers). The study in neonates has recently been finalized and is now in the process of regulatory review in the EU and US.
For the conduct of these studies, the company collaborated closely with a global academic paediatric research network called IMPAACT, the International Maternal Pediatric Adolescent AIDS Clinical Trials Network. This is a worldwide collaboration of researchers, community representatives, and other partners that aims to decrease significantly HIV and HIV-associated infections and to decrease mortality and morbidity due to HIV and HIV-associated infections and co-morbidities among infants, children, adolescents, and pregnant and postpartum women. There were a total of 194 children involved in the clinical research programme and it took 10 years to complete the trials in all age groups.
Thus, this paediatric development programme resulted in adequate formulations (Fig.1) which were fully tested to allow appropriate dosing in children from birth through adolescence, thereby closing a critical gap in treatment options in the HIV-infected pediatric population.
Rewards
In 2007, the European Commission deemed Isentress suitable for a conditional marketing authorisation, which is a means of speeding regulatory approval of a new medicine that shows a positive benefit-risk balance in an area of serious medical need. It includes a legal commitment to generate more comprehensive clinical trial data post-authorisation. Receiving a conditional marketing authorisation triggered an earlier starting time for calculating the periods of both regulatory data protection and Supplementary Protection Certificate (SPC) protection during the products life cycle.
Having satisfied all the conditions set out in the Paediatric Investigation Plan, the company will be able to request a corresponding incentive in the form of a six-month extension to its SPC for Isentress. Such a reward can compensate, to a certain degree, for the additional paediatric development costs and for the earlier expiry date of SPC protection than would otherwise be obtained with a full marketing authorisation. Nevertheless, taking this route would have delayed the regulatory approval and consequently the availability of the product to adult patients.
While global regulations drive paediatric drug development, knowing that a system offers rewards is a source of significant encouragement throughout the process. These rewards could translate potentially into financial returns, buffering the cost, time and effort that are invested in the overall development of the product. They can also help to ensure compliance with post-approval regulatory activities that allow stringent safety surveillance. They can furthermore promote improvements in the quality and manufacturing process until the end of the product life cycle, which is usually longer than the patent protection period. Due to this very long medicine development life cycle, developers have to bear significant uncertainties related to further development cost, safety surveillance and financial risks.
HIV treatment is a rapidly-evolving area and new treatment options become available due to the constant innovation efforts of pharmaceutical companies. The prolongation of the SPC period only happens at the very end of the patent term. While this is usually the time of the highest expected revenue for the company, it is uncertain if the product is still the clinical treatment of choice by the doctor at that time, or if it has been superseded by more effective or innovative treatment options.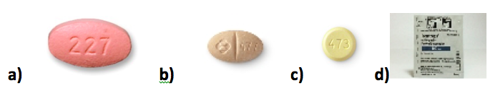
Fig.1 Images representing the available formulations: (a) film-coated tablet (400mg) for adults and children over 25 kg , chewable tablets of (b)100mg with a score and (c) 25 mg for children weighing at least 11 kg , and (d) granules for oral suspension for children at least 4 weeks of age and weighing 3 kg.
[1] UNAIDS, How AIDS Changed Everything, 2015
[2] A.Violari (http://www.nejm.org/doi/full/10.1056/NEJMoa0800971)