Incentives, IP and smaller companies; case story: TiGenix
With over 7,000 medicines in development, new treatments will continue to change patients’ lives, slowing disease progression, avoiding illness and reducing overall costs for healthcare systems. But developing a new medicine is a long, complex and risky process with no guarantees of success. Over the coming weeks, we look at a number of new medicines and the role that pharmaceutical incentives (or IP) have played in their development.
How TiGenix wants to change patients’ lives by improving health outcomes
TiGenix NV (Euronext Brussels and NASDAQ: TIG) is an advanced Belgian biopharmaceutical SME with operations in Madrid, Spain. The company develops novel therapies for severe inflammatory and autoimmune diseases as well as for cardiac diseases by exploiting the anti-inflammatory properties of stem cells. These stem cells are extracted from human adipose tissue from healthy adult donors or myocardial tissue that would typically be discarded during a routine valve replacement operation. Since its founding in 2000, the company has grown to almost 100 employees in 2017.
TiGenix’ lead product, Cx601, is derived from the stem cells of adipose donors. It is being developed for Crohn’s disease patients with complex perianal fistulas in Europe and the USA. Cx601 has successfully completed a European Phase III clinical trial for the treatment of complex perianal fistulas in Crohn’s disease patients and has been filed for approval in Europe; the CHMP decision is expected in 2017.
The impact of the disease on patients, families, healthcare systems and society
Perianal fistulas or anal abscesses are infected cavities filled with pus near the anus. They are a chronic, disabling manifestation and a severely debilitating complication of Crohn’s disease, occurring in 23% to 38% of patients[1]. Crohn's disease affects 0.2% – 0-4% of the population in Europe, and patients are mainly between 15 to 35 years old. Those with the disease are at greater risk of bowel cancer. Patients with perianal fistulas suffer from abdominal pain associated with perianal swelling and fever in the case of abscess formation, and drainage of pus, stool or blood from cutaneous fistula openings. Faecal incontinence and leaking discharge around the rectum may also occur.
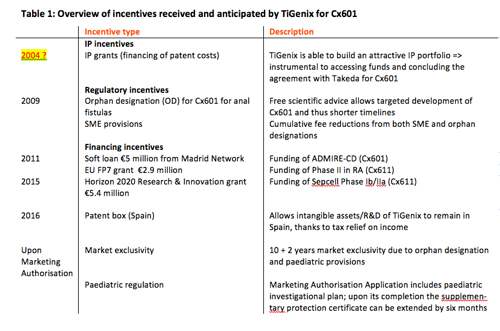
The development pipeline of TiGenix: continued innovation fostered by incentives
The EU pharmaceutical incentives summarized in table 1 supported the development of Cx601. Starting in 2004, clinical development included two Phase II and two Phase III trials, resulting in filing for centralised marketing authorisation (MA) approval in Europe in 2016. No income can be generated for drug candidates, so in the intervening 13 years TiGenix had to cover very high costs to plan and pursue the clinical studies with almost 400 patients. A global Phase III trial with Cx601 started in 2017. It is intended to support a future U.S. Biologic License Application (BLA).
Despite an almost non-existent market, TiGenix agreed to a paediatric investigational plan with the European Medicines Agency (EMA): TiGenix will pursue a clinical trial in patients up to 18 years of age, to ensure that paediatric patients can also benefit from the medical innovation of Cx601.
TiGenix also continues to further expand development activities for innovative and safe treatment options for inflammatory-mediated diseases and other severe conditions. A Phase Ib/IIa trial for the use of a second product candidate, Cx611, in severe sepsis started in the first half of 2017.
IP rights and EU pharmaceutical incentives enable innovation for the cures of tomorrow
TiGenix retains full intellectual property (IP) rights to the technologies and platforms it has developed and utilises. These were instrumental in raising venture capital in 2007 and 2009, accessing debt instruments (venture debt in 2009 and loan facility in 2013), securing partnerships, notably with Takeda in 2016, as well as for accessing the capital markets in Europe several times and listing in the US in December 2016. All these funds have been used for the development and future commercialization of Cx601. Without theseIP rights as securities, it is unlikely that the company could have benefited from appropriate investment to reach the stage where a marketing authorisation application could have been filed.
The innovative nature of its products has also allowed TiGenix to access funding from regional and European entities to fund clinical trials, including a €5 million soft loan from Madrid Network, a €2.9 million FP7 grant and a €5.4 million grant from the European Union under the Horizon 2020 Research and Innovation Program.
EU pharmaceutical incentives have been crucial to driving the development of TiGenix’ lead product, Cx601, as a novel, innovative and safe therapy to improve the health and quality of life of patients suffering from Crohn's disease with complications caused by complex perianal fistulas. The orphan designation (OD) in Europe has allowed TiGenix to benefit from regulatory incentives, including the possibility to seek scientific advice on six occasions during the development of Cx601 at no cost to the company. The combination of OD and Advanced Therapy Medicinal Product (ATMP) status for Cx601 and the EMA designation of TiGenix as SME brings additional incentives. TiGenix benefits from 100% reductions in Marketing Authorisation Application (MAA) fees for administrative services and pre-inspection, as well as 90% reduction of post-authorisation procedures fees, including activities relating to the collection, detection, assessment, monitoring, and prevention of adverse effects (pharmacovigilance).
In addition, as with other small and micro organisations and non-commercial sponsors of clinical trials, TiGenix benefits from a MedDRA licence-fee waiver programme to facilitate electronic pharmacovigilance reporting in the pre- and post-authorisation phase.
With maintenance of the orphan designation at the time of marketing authorisation, TiGenix would benefit from 10 years' market exclusivity that could be extended to 12 years upon completion of the paediatric investigational plan.
[1] Hellers G, Bergstrand O, Ewerth S, Holmström B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut. 1980;21:525–527.