A fact-based analysis of the TRIPS Waiver extension
New data published today reveals the impact of a proposed TRIPS waiver extension to therapeutics and diagnostics - on patient care and on the future of the research-based pharmaceutical industry. WTO members are currently discussing an extension of the TRIPS COVID-19 vaccine waiver to try to increase production of therapeutics and diagnostics and see more patients benefit from treatments. This effectively means removing the Intellectual Property from a company who researched, developed and manufactured treatments, allowing anyone to reproduce it. The answer to COVID-19 therapy access or a nail in the coffin of access initiatives and future pandemic preparedness?
Background to the TRIPS waiver
The pandemic saw industry and partners produce over 15 billion doses of COVID-19 vaccines from scratch in under two years – more than enough to vaccinate the world. Despite this achievement, ahead of the June 2022 WTO Ministerial, advocates of a TRIPS COVID-19 vaccine waiver argued that it was needed to increase production and achieve more equitable global access. Today, just three months later, production capacity for COVID-19 vaccines is being scaled down amid decreasing demand, while only 25% of low-income country populations have been vaccinated. This suggests that the WHO’s 2020 analysis of the key bottlenecks for access were correct: That it is healthcare capacities, distribution barriers and vaccine hesitancy, not IP which is the problem.
Why extend the waiver?
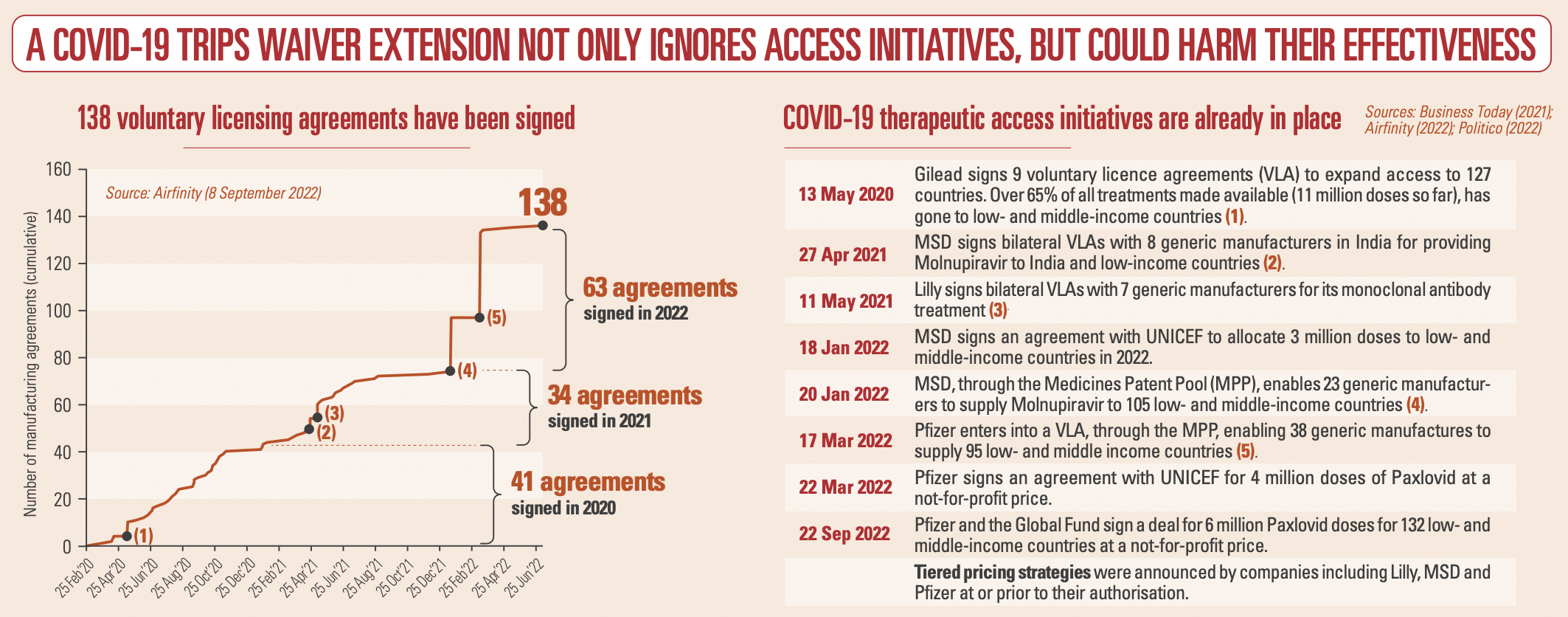
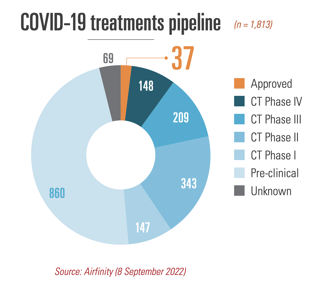
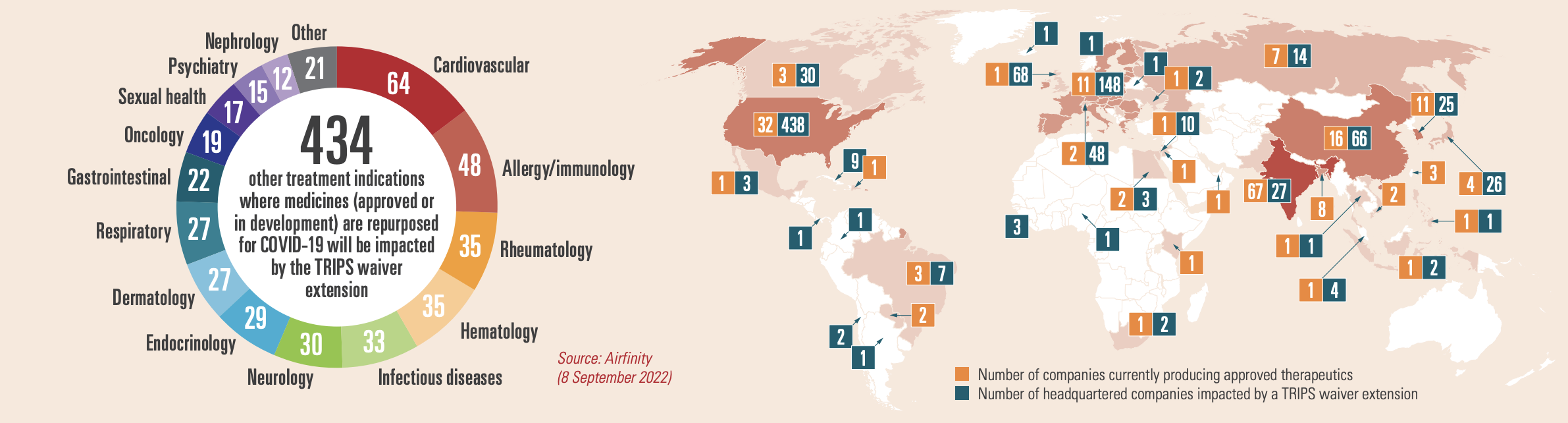
There is no shortage of COVID-19 therapeutics: production exceeds contracted demand for all variants, disease severity levels and in all patient settings. And with global testing declining rapidly, demand for treatments will decrease and become unpredictable. Partnerships with multilateral organisations and 138 voluntary licensing partnerships (Fig. 1) made it possible to ramp up production of therapeutics and, combined with tiered pricing and not-for-profit arrangements for low- and middle-income countries, mean that – for example – 99.9% of Africa and all of South Asia currently have access to the key therapeutics. A waiver would put the legal basis for these initiatives at risk including those under the Medicines Patent Pool (MPP).