Environmental footprint of patient information leaflets (PIL) - How a paper PIL compares to an electronic PIL (ePI) (Guest Blog)
Life cycle assessment (LCA) is a powerful methodology to evaluate the environmental impacts of a product or service over time. An LCA can help answer many questions about where the opportunities for improvement are, how one material or product compares to another, and where there are tradeoffs.
A group of EFPIA member companies operating in Europe, conducted a comparative LCA on the patient information leaflet (PIL) to understand the difference in environmental impacts between a paper PIL and the digital version, an electronic patient information (ePI) document viewed on a smartphone. The study found that an ePI is measurably environmentally preferable to a paper PIL in the impact areas analyzed.
The study was a cradle-to-grave LCA following ISO 14040/14044 standards and was conducted by Long Trail Sustainability (LTS) and peer reviewed by Environmental Resources Management (ERM).
The Challenge
When patients open their medication box, there is always a patient information leaflet (PIL) inside, a bulky paper attachment with information on drug interactions, side effects, storage information, and more. All medicinal products in the European Union must include a paper PIL. In 2021, Japan approved a policy requiring that all PILs be in digital rather than paper format, called an ePI (electronic patient information). An ePI is typically distributed via a QR code on the medication.
Often society views the reduction of paper as an automatic win for the environment, but when considering all aspects of the lifecycle of a paper PIL vs. an ePI viewed on a smartphone, which one will have fewer environmental impacts?
The Approach
Long Trail Sustainability was commissioned to conduct a life cycle assessment (LCA)[1] to provide a comprehensive, scientific method to answer the question.
An LCA measures the material and energy inputs as well as waste and emissions of a product, evaluating multiple environmental impact categories (e.g. climate change, ecosystems, etc.) over the lifetime of the product. The study was cradle-to-grave, including all stages of the lifecycle: raw materials, manufacturing and distribution, use, and disposal. For the ePI, the lifecycle includes the patient’s smartphone device, the energy for data transfer, and the eventual disposal of the smartphone at end of life.
The following functional unit was used for the study: One patient information leaflet (PIL) provided as either a paper PIL or online as ePI, to the 2020 European market. This was a 3.5-gram paper PIL and a 1.958 MB ePI viewed on a smartphone for 10.5 minutes.
Five pharmaceutical companies (Takeda, GSK, Merck, Novartis, and Sanofi) gathered and provided primary data on the paper PIL materials, PIL printing facilities across Europe, PIL packaging, and ePI file size for the study under the direction of LTS. Where primary data was not available, secondary data and literature values were used (e.g., printing data, smartphone data, length of reading time on smartphone). LTS modeled and analyzed the paper PIL and ePI lifecycles using SimaPro LCA software.
The Results
Results indicate that an ePI has between 89% - 98% fewer environmental impacts in the categories studied, shown in Figure 1. A paper PIL has 20 times higher climate change impacts than an ePI.
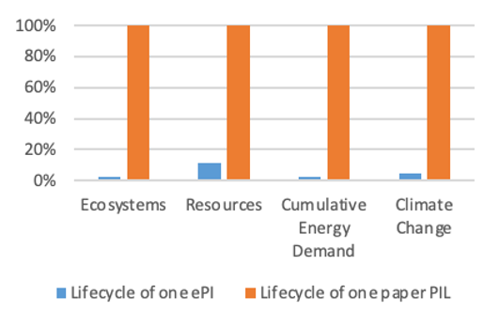
Figure 1: Comparative analysis of one ePI to one paper PIL
When scaling up to a full year of drug sales for four of the pharmaceutical companies, 5.2 billion units, switching to 100% ePI would reduce carbon emissions by 49,507 MTCO2e in one year.
The study made some conservative estimates for the ePI, including an assumption that 100% of ePI would be viewed (QR code scanned by a smartphone), that each person would spend 10.5 minutes reading it, and only a 2.5-year assumption for smartphone lifespan. In one survey, 37% of patients said they always read the PIL, while 52% said they occasionally read it.[2]
The comparative results are considered to have high certainty and to be statistically significant in four impact categories studied, shown in Figure 1. The background datasets used for both the paper PIL and ePI have high variability in the human health and water use categories, causing uncertainty in the human health and water use results. Because statistically significant conclusions cannot be made in these two impact categories, they were removed from the analysis.
The majority of the environmental impacts of the paper PIL come from paper, whereas most of the impacts of the ePI come from the smartphone device.
The primary recommendation from this study is for pharmaceutical companies to switch from using paper PILs to ePI to reduce environmental impacts significantly. If the pharmaceutical companies continue to use some paper PILs, reducing the size of the PIL would reduce the paper needed. Also, maximizing the recycled paper content in the paper PIL would reduce impacts in most impact categories, as found in a sensitivity analysis.
The Value
This document helps answer the question and identify areas for process improvements in a scientific manner to inform the ecosystem present on the European market through this ISO-compliant, critically reviewed life cycle assessment.[3] As industry and consumers strive to lessen the collective impact on the planet, this information helps inform decisions.
LCA Assessment Organization
- Long Trail Sustainability (LTS) works with organizations to integrate life cycle sustainability practices into their business conduct. Whether it’s a broad-spectrum approach or a focused effort geared towards product design, LTS is a skilled consultancy offering LCA and sustainability trainings, ISO-compliant LCA and carbon footprint studies and critical reviews, and high-quality software tools including the world renowned SimaPro LCA software.
LCA Certifying Organization
- Founded in 1971, Environmental Resources Management (ERM) is the largest advisory firm in the world focusing solely on sustainability, offering unparalleled depth and breadth of expertise. ERM creates a better future by helping the world’s biggest brands address today’s sustainability imperatives. This includes developing and implementing sustainability strategies, creating sustainable products and supply chains, developing physical assets sustainably, and integrating sustainability and compliance into daily operations.
[1] Hammar, T., Nilsson, A.-L., & Hovstadius, B. (2016). Patients’ views on electronic patient information leaflets. Pharmacy Practice.
[2] The study follows the ISO 14040/14044 guidelines for LCA. As such it was critically reviewed by a panel of 3rd party experts.
[3] Dodge, S., Bowers, T., Hamilton, M. (2025). Life Cycle Assessment of Patient Information Leaflets: A comparison of paper PILs to the digital counterpart (ePI). Long Trail Sustainability.
