EU Regulatory Network - New Architecture for a New Era (Guest blog)
Imagine you built a house. It was not big, but sufficient for what you needed at the time. Over the years, your family grew, and that same house was not sufficient anymore. You realize you need to renovate so that it fits your current and future needs.
When we look at the current EU regulatory system which was established 27 years ago, and compare this with the house analogy, we see that structures, systems and processes have been added over time to address specific needs without revisiting the overall architecture. The EU has grown from a family of 15 to 27 Member State and moving within the framework is slow and difficult. The framework has become a complex and inefficient construction.
New science and technologies are evolving fast with integrated solutions for personalized healthcare. However, conventional processes coupled with complex governance within the EU regulatory network make it challenging for innovative therapies to rapidly make their way to patients. Europe is lagging behind other leading regulators in terms of agility and speed. The time to act is now[1] [2]: With the European Commission’s evaluation of the pharmaceutical legislation as part of the EU pharmaceutical strategy[3], we have an unprecedented opportunity to reconstruct the EU’s regulatory framework, processes and governance. Every house needs renovation at some point. What should the new structure look like so that it will fit current and future needs?
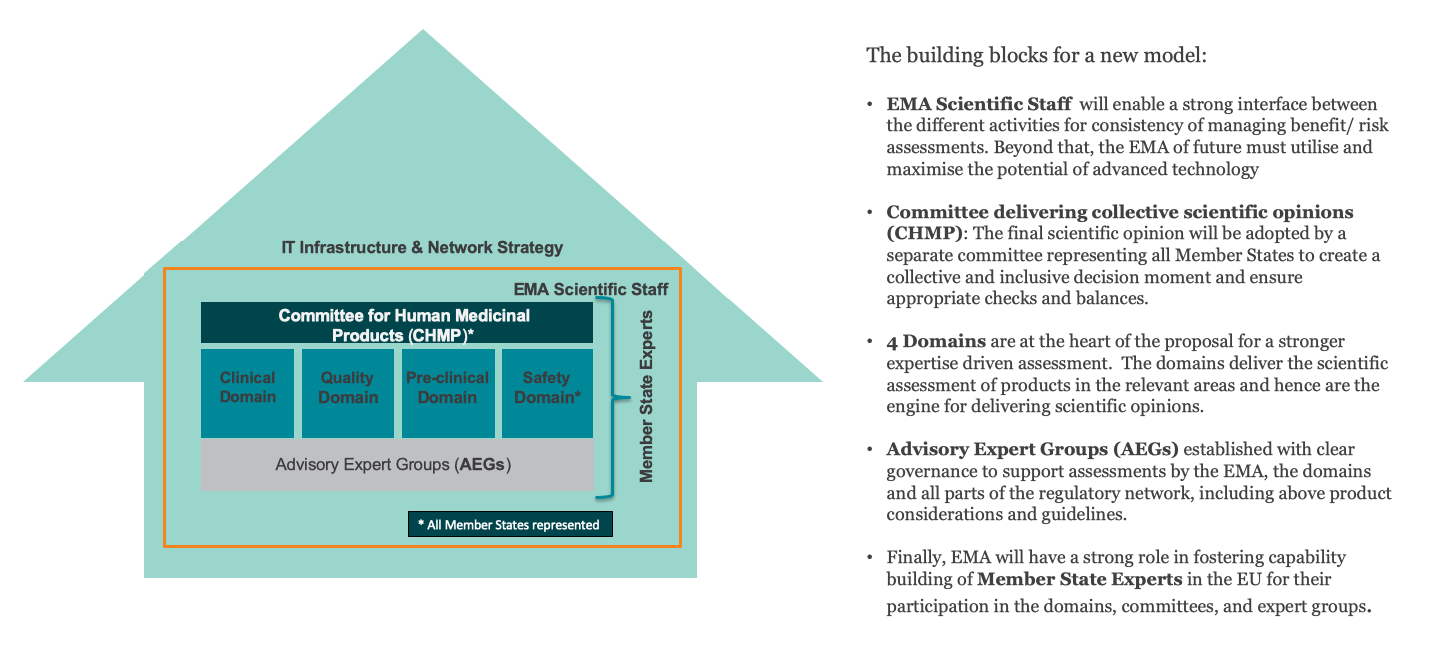
EFPIA’s overall goal is to reinforce expertise-driven assessment and enable a more agile centralized authorization framework by facilitating Member States ability to bring forward their expertise and by removing unnecessary, burdensome interfaces between Committees and the European Commission.
To achieve this, EFPIA proposes the following renovations:
- High-quality assessments need to be ensured and further strengthened by revising the governance framework, enabling the best expertise to be brought forward by the EU Member States in the areas where they can best contribute.
- The need for agility in the system requires us to rethink the current outdated committee and working parties structure for agility and efficiency gains.
- To address any resource gaps related to both current and future workload, as well as the implementation of forward-looking strategies, it will also be crucial to rethink funding and resourcing mechanisms for EMA and the Network.
We spend years developing a drug, bringing our best experts to the table. Given the complexity of drug development, we need to move away from a few formalized meetings with regulators on scientific advice to more frequent dynamic interactions with best scientific and regulatory experts to create clarity on drug development expectations in a dynamic environment. Regulatory processes and governance of EMA and the Network need to support this, ultimately enabling an informed and streamlined assessment of marketing authorization applications.
The EU Regulatory Network response to the COVID-19 pandemic demonstrated that European stakeholders could work and collaborate together in order to significantly accelerate the regulatory approval process. We sense a strong will from all actors in the system to leverage and build on this experience, and at the same time a desire for urgent renovation. Through EMA and the EU Regulatory Network, Member States have been collaborating intensively for over 27 years. Now may be the right point in time to bring the trust established to another level.
Patients and healthcare professionals are waiting for new treatments; would they not expect an urgent renovation of the current house?
[1] https://efpia.eu/media/636486/improving-regulatory-timelines-to-optimise-patient-access-to-innovative-oncology-therapies-in-europe.pdf
[2] https://www.efpia.eu/media/636564/evidence-mix_final-9-dec-2021.pdf
[3] https://www.efpia.eu/about-medicines/development-of-medicines/regulations-safety-supply/regulatory-road-to-innovation/

