Everyday counts: time to patient access to innovative oncology therapies in Europe
We live in times when speed of innovation for cancer patients is unprecedented: In 1996, a physician had only 4 medicines to treat lung cancer, in 2016 there were 19 different medicines available, as an IQVIA report showed in 2017.[1] If we did the same assessment today we would likely be concluding that there are many more advanced novel treatment options available.
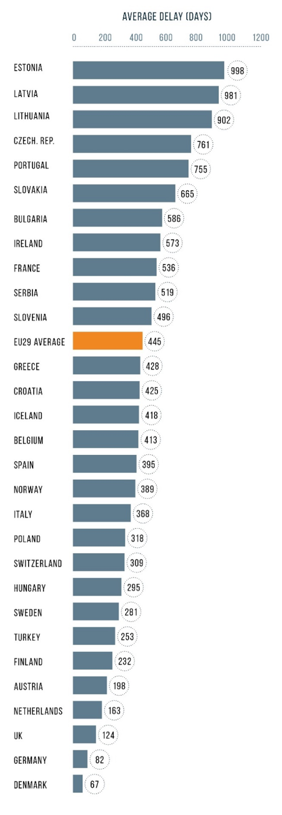
However, there is no value from innovation if patients for whom it is intended, cannot have access to it. To illustrate this, patients in the Czech Republic wait seven times longer than patients in Denmark or Germany until a new cancer treatment is publicly reimbursed. [2] In the Netherlands, 9 out of 10 new cancer medicines with EMA marketing authorization are available. In Poland only 2 out of 10 new treatment options are available. Such inequalities are also reflected in the outcomes seen. For example, the five-year net survival rate of colon cancer is 63.1 % in the Netherlands, 10% higher than that in Poland. Similarly, the five-year net survival rate of breast cancer is 86.6% in the Netherlands vs. 76.5% in Poland.[3] While screening and early diagnosis play an important role in these differences, access to novel treatments is not negligible.
While we know at least something about variances in time and availability, little is known about the reasons behind these delays or other limitations to availability. And even when medicines are reimbursed, we often don’t know whether they have entered clinical practices. That’s why the Oncology Platform of the European Federation of Pharmaceutical Industries and Associations (EFPIA) wants to address these questions and have decided to initiate a project named Time to Patient Access.
The ambition of the “Time to Patient Access” project is to bring together diverse stakeholders in countries across Europe to jointly define the project approach, identify factors which cause delays, and co-create practical solutions that could accelerate time to patient access. These stakeholders include patient organizations, regulators and policy makers, health technology assessment (HTA) bodies, payers, healthcare professionals, leading health economists, and the industry.
To achieve this objective, we are all working together to answer the following questions:
- What factors delay access to new cancer medicines across Europe?
- Which best practices and what recommendations can improve time to patient access?
The project also assesses the foregone health impact caused by delayed or denied patient access and gives an estimate on the positive impact which could be generated by improvements in time-to-patient access.
Supported by a consortium comprising Vintura agency, ASC Academics, and Hague Corporate Affairs, the project will achieve its objective by:
- Applying an unbiased, multi-stakeholder perspective
- Ensuring an academic robustness of research methodologies
- Focusing on tangible solutions with impact, co-created with all relevant stakeholders.
As of today, through interviews and / or face-to-face meetings, the project has received valuable insights and expert contributions from many stakeholders including patient organisations, HTA bodies, regulators and policy makers, payers, leading health economists, and the industry.
The project will continue to run until January 2020. Stakeholders with an interest to share your views and help co-create tangible solutions are welcomed to join us (email: cJansen@vintura.com). All insights and contributions are welcomed!
Figure 1 Time between EMA approval and reimbursed access to new oncology medicines. Source: EFPIA W.A.I.T. Indicator, 2018
[1] M. Aitken, M. Kleinrock, and S. Kumar, “Global Oncology Trends 2017. Advances, Complexity and Cost,” QuintilesIMS Inst., no. June, pp. 1–40, 2017
[2] EFPIA Patient W.A.I.T. Indicator 2018 survey. “Patients W.A.I.T.” stands for Patients Waiting to Access Innovative Therapies. The INDICATOR provides a benchmark of the rate of availability and waiting times in European countries;
[3] Allemani, C., Matsuda, T., Di Carlo, V., Harewood, R., Matz, M., Niksic, M., et al., Global surveillance of trends in cancer survival 2000-2014.