From theory to therapy – MSD Policy Passport on Pharmaceutical Innovation (Guest blog)
Policy makers are in the driving seat. Their decisions play a key role in the creation and sustainability of Europe’s research and innovation ecosystem. It is thanks to these policies that in 2021 pharmaceutical companies have invested an estimated €42,5 billion in R&D in Europe
Over the last sixty years, pharmaceutical companies have made a phenomenal contribution to improve healthcare and patients’ lives. Since 2001 alone, 1018 innovative medicines and vaccines have been launched, tackling a range of conditions for both infectious and chronic diseases. [1]
A key measure of the contribution of pharmaceutical innovation is the continuous progress in life expectancy we have experienced during the last decades. In the 2000-2009 period alone, innovative medicines are estimated to have contributed up to 73% of the increase in life expectancy at birth after accounting for other factors. [2]
Policy makers are in the driving seat
There is nothing obvious or simple in the development of medicines or vaccines. Pharmaceutical innovation is at the junction of scientific advances, unmet medical needs, and entrepreneurship. As a result, it calls for a sophisticated policy framework that cuts across three main pillars: Investment in fundamental research, sustained efforts to improve population health through developed healthcare systems, and the implementation of pro-innovation industrial policies, such as intellectual property rights.
Still today, only a handful of countries and regions remain the overwhelming source of novel medicines and vaccines. What they have in common is their continuous investment in these three main pillars. However, science, healthcare, and industrial policies come with their specific goals and instruments, eg, access to affordable medicines, attracting R&D investments, increasing pharmaceutical exports, etc. These goals are not exclusive but explain the complexity of designing pharmaceutical policy.
This level of complexity, if not fragmentation, results in a clear challenge for policy makers. How these policies are shaped, and the decisions taken by policy makers and regulators, can either accelerate or deter R&D investments as well as patients’ access to innovative medicines, to take two key policy objectives.
Across this policy framework, policy makers are in the driving seat. Their decisions play a key role in the creation and sustainability of Europe’s research and innovation ecosystem. Put into perspective, it is thanks to these policies that in 2021 research-based pharmaceutical companies have invested an estimated €42,5 billion in R&D in Europe. [3]
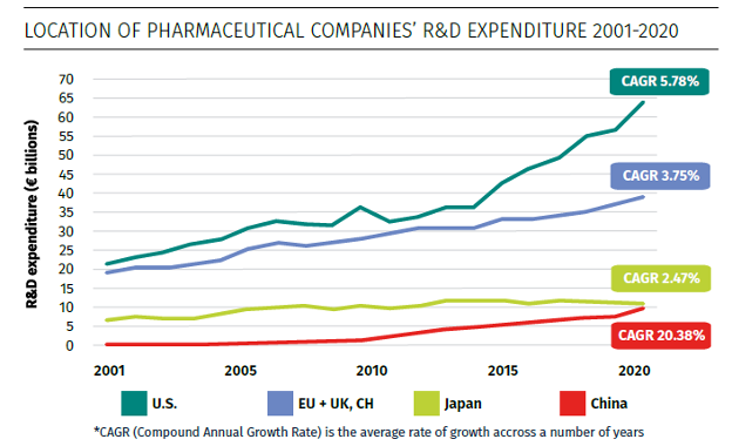
Source: Charles River Associates, Factors affecting the location of biopharmaceutical investments and implications for European policy priorities, Figure 1, November 2022.
Your visa to pharmaceutical policy
Taking stock of this challenge and the key role played by research-based pharmaceutical companies for patients, health systems and economy, we developed the MSD Policy Passport on Pharmaceutical Innovation. This document is intended to provide policy makers with a roadmap of the drivers and critical policies that support pharmaceutical innovation. Our Policy Passport addresses key aspects of pharmaceutical R&D, sheds the light on numerous issues such as affordability and access, and overall highlights the critical role played by government policies in creating the right conditions to sustain pharmaceutical R&D efforts and improve access to medicines.
As the EU is embarking on a comprehensive reform of its pharmaceutical legislation, we hope that our Policy Passport will help advance the collective thinking and inform the debates about how to ensure that research-based pharmaceutical companies, such as MSD, can continue to translate science into medicines for European patients. See more at www.msd.com.
[1] Pharma R&D Annual Review 2023 Supplement: New Active Substances Launched During 2022, Citeline, May 2023. https://www.citeline.com/en/resources/clinical/pharma-rd-annual-review-2023-nas-supplement
[2] Lichtenberg F., Pharmaceutical innovation and longevity growth in 30 developing OECD and
high-income countries 2000–2009, Health Policy & Technology, 2012. http://dx.doi.org/10.1016/j.hlpt.2013.09.005
[3] EFPIA, The Pharmaceutical Industry in Figures – Key Data 2023 https://efpia.eu/media/rm4kzdlx/the-pharmaceutical-industry-in-figures-2023.pdf

