Moving at the speed of science to beat childhood cancers
15.02.22
15 February marks International Childhood Cancer Day, providing an opportunity to raise awareness and emphasize the importance of supporting research to give all children with cancer the best chance to live their dreams.
The research and development of new medicines for children is so fundamentally important to us all - for every child, for every family affected by childhood illness, for our clinicians, healthcare systems and for society as a whole. It is why our industry is fully committed to integrating paediatric research in its medicine development programs.
Over the last decade we have seen exciting new treatments and even cures for diseases impacting on children such as HIV, Hepatitis C and rheumatoid arthritis. These new treatments have been enabled by rapidly advancing science and the EU Paediatric Regulation which created a framework to support the development of new medicines for children. Since its inception in 2007, more than 290 new medicines and indications for treating children were approved by the EMA[1].
While thanks to advances in cancer care, 80% of children now survive 5 years or more[2], we know that more can be done. Cancer still being the leading cause of death by disease among children in Europe, we can’t stop here.
Towards a ‘child-centric’ approach
In the current system, every new treatment that is developed for adults must also be developed for children or so-called “paediatric patients”. However, if the corresponding disease does not exist in children, a waiver can be granted.
This means that in its current form, the Paediatric Regulation does not incentivize paediatric-focused development. The upcoming review of the Regulation provides an opportunity to evolve from an ‘adult-centric’ approach to a ‘child-centric’ approach.
To achieve this goal, we are proposing to add to the Regulation the possibility of having Paediatric Investigation Plans (PIPs) that are based not on the adult indication, but on a paediatric unmet medical need that the treatment can address based on its Mechanism of Action (MoA). This would mean that a drug developed for an adult disease (such as an adult-only cancer), may also be studied in a childhood disease because both diseases have the same cause, and the treatment may work for both.
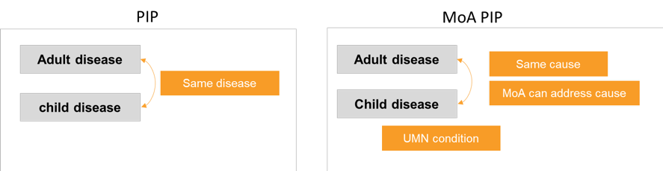

We believe that in this way MoA PIPs will leverage scientific advances to ensure the development of medicines for children that better target paediatric unmet needs. Such an MoA PIP would be filed by a company when there is a broadly recognised paediatric unmet medical need that the medicine can plausibly address. This means that enough knowledge of the paediatric disease is available to show that the mechanism of action of the treatment is relevant to the disease. Enough early evidence should also be available to plan and carry out studies in children that are likely to lead to useful results. This approach will be particularly suitable in oncology, leveraging the dynamic research and development in adult cancer to address the needs of children.
Adding the MoA PIPs as a further option among the existing obligations, requires progressive dialogue with the European Commission and other partners. We are looking forward to working with all partners to create a regulatory environment centred around children’s needs and scientific progress.
[1] https://www.frontiersin.org/articles/10.3389/fmed.2021.593281/full
[2] https://worldspanmedia.s3-eu-west-1.amazonaws.com/media/siope/PDF/the-siope-strategic-plan.pdf
