The Future of Patient-Centered Oncology Care in Europe
This week sees cancer experts from all over the world descend on Madrid for ESMO2017, the European Society of Medical Oncology’s annual congress.
Improvements in both cutting-edge science and treatment outcomes are changing the profile of cancer from an acute to a chronic disease, transforming cancer care and most importantly increasing the proportion of cancer survivors. For European patients diagnosed in 2012, over 66,000 more will live for at least five years after diagnosis compared with if they had been diagnosed a decade earlier.1 However, new innovations are often considered to be costly and several organisations have stated concerns with regard to the budget impact of new medicines and the sustainability of health systems in Europe.2
More people are being diagnosed with cancer but services are helping patients live longer and with a better quality of life
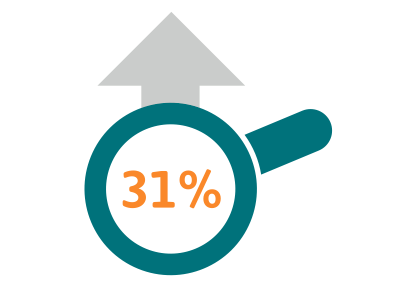
Cancer incidence in Europe increased by 31% between 1995 and 2012 2.6 million people were diagnosed with cancer in 1995, rising to 3.4million people diagnosed with cancer in 20123

For European patients diagnosed in 2012, over 66,000 more will live for at least five years after diagnosis, compared with if they had been diagnosed a decade earlier4
In light of these developments in cancer care and of the need for a fresh European conversation, 15 of EFPIA’s member companies* have funded a collaborative project to host a series of roundtable discussions with the objective to proactively discuss the future of cancer care in Europe.
In parallel, the Swedish Institute for Health Economics has been working on a new Comparator Report on Patient Access to Cancer Medicines in Europe 2016, which analyses the impact of cancer and the level of patient access to new cancer medicines across Europe.
Acknowledging the challenges but also the opportunities for more individualised treatment and more options leading to improved outcomes, it is important that all relevant stakeholders, from policy makers to industry, work together to find jointly agreed and commonly beneficial solutions to ensure that the policy environment is adapting to maximise the benefits and minimise the concerns associated with these new oncology treatments.
What has the impact of cancer medicines been on patients and health services?
Scientific advances, leading to better cancer treatment, deliver an immense human benefit
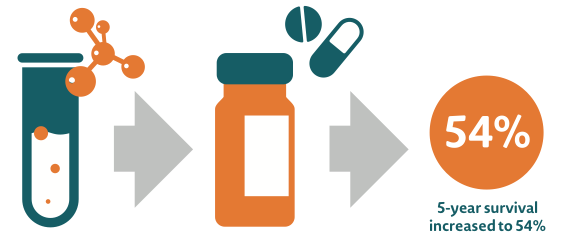
Although more people are being diagnosed with cancer, advances in treatments and services including screening and diagnostics, are helping patients live longer and with a better quality of life. Furthermore, new medicines are helping people of all ages live with, and increasingly, survive cancer.5
If current trends continue, cancer is set to become the biggest cause of disability in Europe
![Disease burden of the top 5 disease groups in Europe, 2000-2012 [10]](/media/219928/esmo3.png)
Disease burden of the top 5 disease groups in Europe, 2000-2012 [10]
Better treatment and improved survival means people are able to return to work, playing an active role in society and the economy. It has also enabled people beyond working age to live longer, more active and fulfilling lives.8
Investment in cancer medicines delivers an overall economic benefit to society. Furthermore, spending on new cancer medicines has not threatened the financial stability of economies so far and should not be seen as a threat for the future.9
Cancer services are treating more people for longer and they are doing so with a relatively stable share of the health budget. New medicines have not been the primary driver of increases in expenditure on cancer drugs. This is a consequence of more effective treatments, with fewer side effects. Similar patterns have occurred for asthma and cardiovascular diseases after effective new treatments were made available.11
More new medicines with fewer side effects have also helped support efficiencies in other parts of cancer care, reducing hospital stays and enabling people to receive care at home or in the community. This in turn has enabled investment in new treatments. This is a success story.12
As older cancer medicines lose patent protection, competition from generics/biosimilars is key to generate savings, contribute to the sustainability of health systems and foster innovation. Such innovative, more effective cancer treatment will be critical to helping society manage the cost of cancer in the future.13
There is no room for complacency on cancer anywhere in Europe. More than one in four deaths in Europe are due to cancer,14 but cancer only accounts for just over six per cent of health expenditure.15 The number of cancer diagnoses increased by nearly a third between 1995 and 2012 and will increase further due to the ageing population and lifestyle factors.16,17 Cancer is set to become the biggest cause of death and disability in Europe.18
Progress remains painstakingly slow in some cancers. We must redouble our efforts to ensure that scientific advances occur in all cancers. Cancer is complex and clinicians need access to as many treatment options as possible so they can tailor treatment to best meet the individual needs of their patients.19
There is more that can be done to ensure that everyone gets the best treatment for them
When it comes to cancer drugs, countries in Eastern and Southern Europe spend about one third the level of countries in Western Europe and this pattern does not appear to be changing over time.22
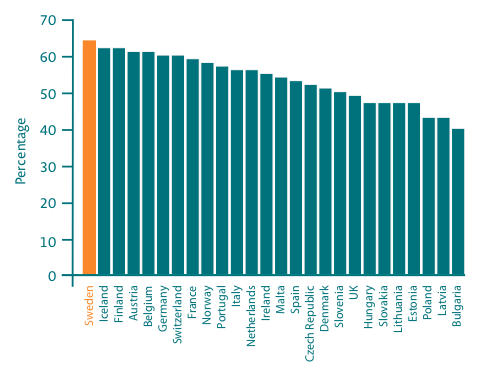
5-year relative survival for all cancer varies across Europe from 40% in Bulgaria to 64% in Sweden20
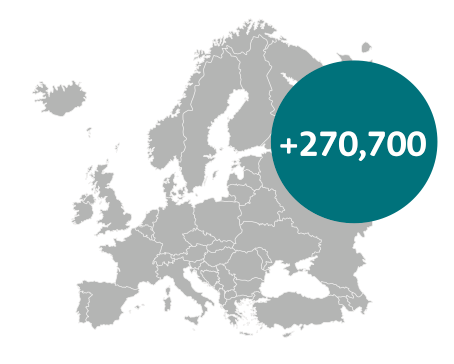
If every country were to achieve the survival rates of Sweden, then an additional 270,700 people would survive for at least five years21
Despite new cancer medicines being a relatively small driver of overall cancer cost, some countries are more rapid adopters of new treatments than others.23 Patients in some countries face long delays in gaining access to effective new cancer drugs.24
Improving access to cancer treatments does not have to require significant increases in costs. Countries with similar levels of cancer spend currently have very different levels of usage of cancer drugs.25
There is a six-fold difference in spending on cancer across Europe and this pattern does not appear to be changing over time26

Countries in Eastern and Southern Europe spend around one third the level of countries in Western Europe and this pattern does not appear to be changing over time27
Although survival is improving in every European country, there are still significant inequalities.28 Cancer survival is influenced by a range of factors, but countries that are higher users of newer cancer treatments often report better outcomes.29,30 Variations in access to treatment, combined with inequalities in survival, suggest that not all people are accessing the best quality of treatment and care.31,32
Patients in some countries face long delays in gaining access to effective new cancer drugs
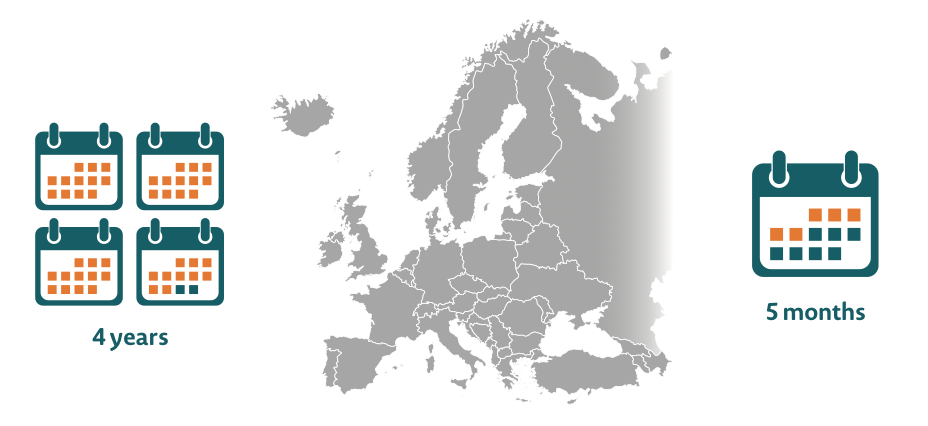
Delays in patient access to innovative new treatments can range from five months to up to four years across Europe33
We want every patient who could benefit to be able to receive the most appropriate cancer medicine for them.34 The ageing population means that the cost of delivering health services, including cancer, will rise.35 We need to find a way to manage this pressure and enable patients to benefit from effective new treatments. We are keen to work in partnership with countries to identify what can be done to find efficiencies and fund improvements in cancer care.36
Enabling all patients to access the best treatment requires clinicians to be aware of different options, services to be organised to deliver treatment efficiently and effectively and medicines to be made available at an affordable price. EFPIA members are supporting health services on all of these issues.
We recognise that differences in access to treatment in Europe are partly caused by variations in wealth and we want to help countries address this.38 Yet parallel trade and international reference pricing limit the scope for price differentiation according to a country’s ability to pay.39 We want to discuss how we can collectively address these issues.
Thanks to scientific progress, more people are living longer and better quality lives with cancer. However, more cancer diagnoses and increased need for treatment create funding challenges

EFPIA members stand ready to play their part in enabling fair and sustainable access to the best cancer medicines today and in the future37
We acknowledge the affordability challenges faced by healthcare systems.40 We all have a responsibility to act. Regulators have responded to developments in science and the needs of patients to introduce greater flexibility to the way in which they assess new medicines.41
There are some encouraging examples of countries pioneering new approaches to access, including managed entry agreements, early HTA advice and linking payment to outcomes delivered.42 However, gaps in data can make these schemes challenging. Developing new access models is a complex but necessary task that requires action from many different stakeholders. Our members want to work with governments to make this happen.43
References
1 Calculations based on incidence figures quoted in Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 - page 16: 2000-2002 5-year survival rate in all cancers in Europe: 51.55% 2000-2007 5-year survival rate in all cancers across Europe: 54%; Incidence in 2012 in Europe: 2,707,000
2 WHO Europe (2015), Access to new medicines in Europe; OECD (2015), Pharmaceutical expenditure and policies: Past trends and future challenges. DELSA/HEA(2015)6, p. 38.
3 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016- page 4: Cancer incidence increased by 31% from 1995 to 2012 (external reference: Ferlay J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374-403).
4 Calculations based on incidence figures quoted in Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 - page 16: 2000-2002 5-year survival rate in all cancers in Europe: 51.55% 2000-2007 5-year survival rate in all cancers across Europe: 54%; Incidence in 2012 in Europe: 2,707,000
5 EFPIA, Medicines costs in context, 29.03.2016 - page 7: From 2000 – 2009, an improvement in population weighted mean life expectancy was 1.74 years. Innovative medicines are estimated to have contributed 73% of this improvement (external reference: Lichtenberg: Pharmaceutical Innovation in 30 developing OECD and high-income countries, 2000-2009 (2012)
6 EFPIA, Medicines costs in context, 29.03.2016 - page 6: New therapies have contributed to significant declines in cancer death rates around the world since its peak in 1991. Today 2 out of 3 people diagnosed with cancer survive at least 5 years (external reference: PhRMA 2016 Prescription Medicines: Costs in Context)
7 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016- pages 4 and 16: the ehe l. ncidence increased in Eovide positive value in nin countries, but the US lags in health gains per dollar spent, 2016 nt : : estimated cancer incidence was 2.707 million (external reference: Ferlay J et al, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11: International Agency for Research on Cancer; 2013); The average 5-year relative survival rate for all cancer types combined was 54% in Europe for cancers diagnosed between 2000 and 2007 (external reference: De Angelis R, et al Cancer survival in Europe 1999-2007)
8 EFPIA, Medicines costs in context, 29.03.2016 slide 34; Over 75% of people of working age now return to work after cancer treatment, (external source: Amir Z 2009 Cancer Survivorship and employment Occup Med, studies carried out in the US and in the North-West of England)
9 AstraZeneca, comparative healthcare financing trends in Europe: A retrospective and forward-looking view, 2016: European countries experienced a decline in real-terms growth in public healthcare expenditure over 2010-14 compared to the pre-crisis trend & HealthAffairs, Cancer drugs provide positive value in in countries, but the US lags in health gains per dollar spent, 2016 ehe l. ncidence increased in Eovide positive value in nin countries, but the US lags in health gains per dollar spent, 2016 nt & Spending on cancer medicines across the EU represents only 1% of overall healthcare spending and only ¼ (27%) of Total Spending on Cancer Care, EFPIA, Medicines costs in context, 29.03.2016
10 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016- page 9: The epidemiological development in other leading causes of death, in particular the decline in cardiovascular diseases in recent decades, influences cancer mortality figures. Since more people reach an advanced age, this leaves more people at risk of getting cancer and eventually also die from cancer (external reference: Honoré BE et al, Competing Risks Models and the War on Cancer. Econometrica. 2006;74(6):1675-98)
11 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 - page 145: The introduction of preventive measures and treatments reduced cardiovascular mortality by 50% over the last decades
12 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – Executive Summary iv: the direct health cost of cancer has remained around 6% of over the last 20 years. A major contributing factor is the shift from in patient to ambulatory and home care and the development of less toxic cancer medicines
13 EFPIA, Medicines costs in context, 29.03.2016 - page 7: From 2000 – 2009, an improvement in population weighted mean life expectancy at birth was 1.74 years. Innovative medicines are estimated to have contributed to 73% of this improvement (external reference: Lichtenberg: Pharmaceutical Innovation and longevity growth in 30 developing OECD and high-income countries, 2000-2009 (2012)
14 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page iv: In 2012 the burden of cancer disease was second greatest with more than one in four deaths due to cancer
15 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page iv: the direct health cost of cancer has remained more or less flat around 6% of total health expenditure over the last 20 years
16 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016- page 4: Increase by 31 percent in incidence from 1995 to 2012 (external reference: Ferlay J et al Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013)
17 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016- page 4: The elderly account for a growing share of the total population. It is clear that the risk of getting cancer increases at old age, and thus a growing share of elderly gives rise to more cancer cases. (external reference: Eurostat. Population on 1 January by five years age group and sex [demo_pjangroup] [January 22, 2016]. Available from: http://ec.europa.eu/eurostat/.
18 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016- page 9: The epidemiological development in other leading causes of death, in particular the decline in cardiovascular diseases in recent decades, influences cancer mortality figures. Since more people reach an advanced age, this leaves more people at risk of getting cancer and eventually also die from cancer (external reference: Honoré BE et al, Competing Risks Models and the War on Cancer. Econometrica. 2006;74(6):1675-98)
19 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 73: Less than 2% of human diseases are caused by one gene (monogenic), the rest are caused by multiple genes or by changes in the proteins they encode
20 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016- page 16: The [survival] rates varied from 40% in Bulgaria to 64% in Sweden (external references: De Angelis R et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15(1):23-34
21 If every European country achieved the outcomes achieved in Sweden in 2012, then an additional 270,700 people every year would survive for at least five years, figures taken from Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016, page 16
22 Bengt Jönsson et al.,Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 141: Countries in Eastern and Southern Europe, with low GDP per capita, have sales at around 1/3 of sales in countries in Western Europe, both in 2005 and in 2014
23 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 94: The newest drugs make up only 8% of the total average sales, with the higher share in richer countries; Access to cancer drugs varies in Europe and is mainly related to the countries’ economic status
24 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 101: Poland is worst in terms of level of uptake, with population-standardized sales being at best 5 times lower than France and 3 times lower than Germany or Sweden for drugs that have been available for a longer time
25 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 39: despite similar per capita spending on cancer in Denmark and Italy, the survival rates for prostate cancer in Denmark are 20% points lower than in Italy. Poland and France have similar survival rates for lung cancer but spending is 3 times higher in France than in Poland
26 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page iv
27 Bengt Jönsson et al.,Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 141: Countries in Eastern and Southern Europe, with low GDP per capita, have sales at around 1/3 of sales in countries in Western Europe, both in 2005 and in 2014
28 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 61: Between 1990 and 2007 there was a steady increase in cancer survival rates in all countries, yet disparities remain between wealthier countries with higher survival rates and poorer countries with lower survival rates
29 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 16: There is a rather clear pattern of wealthier countries to record higher survival rates, whereas poorer countries record lower rates
30 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 144: Low national income and health care spending per capita are major obstacles for access to new cancer drugs
31 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 141: Countries in Eastern and Southern Europe, with low GDP per capita, have sales at around 1/3 of sales in countries in Western Europe, both in 2005 and in 2014
32 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 61: Between 1990 and 2007 there was a steady increase in cancer survival rates in all countries, yet disparities remain between wealthier and poorer countries
33 Europe Economics, External Reference Pricing, 1 July 2013, page 16, accessible at: http://www.europe-economics.com/publications/external_reference_pricing_-_final_report.pdf
34 EFPIA, Medicines costs in context, 29.03.2016-page 16: Today more than 7000 medicines are in developments around the world targeting areas of high unmet need (external resource: Health Advances analysis, Adis R&D Insight Database)
35 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 4: The elderly account for a growing share of the total population, e.g., the share of people aged 65 years and older increased from 15 to 18: Adapted from van Harten et al sight Database) ts around the world targeting areas of high unmet need (external resource: Healt (external reference: Eurostat. Population on 1 January by five years age group and sex [demo_pjangroup] [January 22, 2016]. Available from: http://ec.europa.eu/eurostat/.)
36 EFPIA, Medicines costs in context, 29.03.2016-page 48: Combination of generic price erosion and price regulation resulted in a 24% decline in medicine prices versus a 30% rise in consumer prices in Europe from 2000 through 2013, (external reference: Health Advances analysis; EFPIA 2015 Sustainable Healthcare Systems Compendia analysis of various OECD databases (accessed in April 2015)
37 EFPIA, Medicines costs in context, 29.03.2016-page 30; Over $1,100 bn worldwide pharmaceutical R&D since 2006 and another $ 900 bn expected investment from 2015 – 2020 (external reference: Health Advances analysis, EvaluatePharma 2015 World Preview; Battelle 2014 Global R&D Funding Forecast)
38 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 169: Figure 78 (external source: Adapted from van Harten et al)
39 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 144: Parallel trade and international reference pricing limits the opportunities for price discrimination
40 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 42: Figure 15
41 EFPIA, Medicines costs in context, 29.03.2016-page 59: Through the IMI 2, a joint undertaking between the European Union and the pharmaceutical industry association EFPIA, a €3.3 billion budget for the period 2014-2024 has been established, (external source: Health Advances analysis; 1IMI website; 2EFPIA 2014 Annual Report)
42 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 164: Early HTA and joint HTA/regulatory advice are important new policy instruments and adaptive pathways model may also provide relevant data for HTA and payer decisions in close collaboration with regulators (external reference: European Medicines Agency, Best Practice guidance for Pilot EMA HTA Parallel Scientific. 2014)
43 Bengt Jönsson et al., Comparator Report on Patient Access to Cancer Medicines in Europe Revisited, 2016 – page 170: EFPIA has taken an initiative to provide more clarity and uniformity of MAA in order to assist local affiliates to go into discussions about the design of such agreements