Podcast: Why is a therapeutics waiver extension different to the vaccine waiver?
The current discussions at the WTO centre around a possible extension of the TRIPS waiver – approved for COVID-19 vaccines in June 2022 – to therapeutics and diagnostics for the disease. This proposed waiver extension would make it easier for compulsory licencing to be used for production of therapeutics, including the waiving of patent obligations. This would entail a significant expansion from the original, narrower vaccine scope. So what is the ultimate objective of such a waiver extension? What are the potential benefits, as well as the drawbacks?
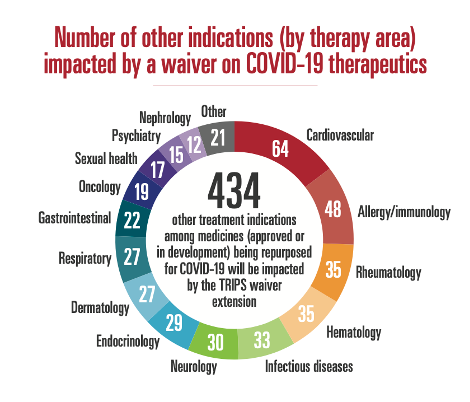
The TRIPS waiver extension proponents argue that an extension will support the ramp-up of therapeutics production and improve access to such medicines, especially in low-income countries. While the goals of ramping up production and improving access are valid ones, not only is a waiver extension not needed, it is also not the way in which these goals can be meaningfully achieved.