‘TEE’ system will revitalise antibiotic research and save money for Europe, new evidence shows
New research published today could pave the way for a permanent solution to the “broken economic model” for researching and developing new antibiotics in Europe. New and novel antibiotics are urgently needed to help tackle the global health threat of antimicrobial resistance (AMR), as infections become more resistant to current treatments.
The figures support the implementation of a Transferable Exclusivity Extension (TEE) proposal – showing it to be the most credible and workable solution for driving antibiotic research in Europe. The system could bring two new antibiotics a year to patients over the next decade, preventing some of the 400,000 deaths associated with AMR every year in the EU.
TEE works by providing the developer of an eligible, novel antibiotic a voucher that can be used to extend the exclusivity of one of its own medicines for a period of time, or sold to another company, thereby paying for successful antibiotic research.
The cost/benefit analysis by Charles River Associates for EFPIA demonstrates the potential benefits – clinical and economic – and allays fears that TEE would prove expensive to Member States. In fact, the research shows that it would save individual countries hundreds of millions of Euros and provide a long-term, sustainable solution to revitalise the antibiotic pipeline.
For the first time the research quantifies the net positive benefits that TEE can deliver: This includes the clinical benefit to patients of treating life-threatening hospital-based infections like sepsis, and complex infections including severe UTIs, as well as the vast benefits that antibiotics play in supporting and enabling surgical and medical interventions. It also includes the benefits of reducing transmission of infections among the population, the insurance value of having treatments in case of major incidents and productivity gains from enabling patients to get back to work.
The report models TEE use in six countries – chosen to give an accurate reflection of the different medicines markets across the EU27*. The table shows the cost savings generated for a new antibiotic incentivised by the TEE over the next decade.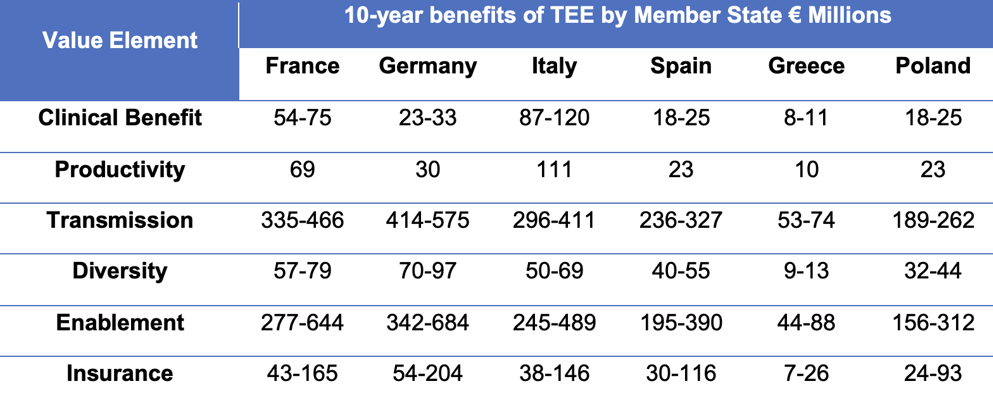
Concerns have been raised that TEE could lead to increased costs from lost genericization of other medicines. However, the report highlights the significant difference between the cost-savings delivered by TEE (above) and cost to healthcare systems of extending the exclusivity of another product using the voucher (below):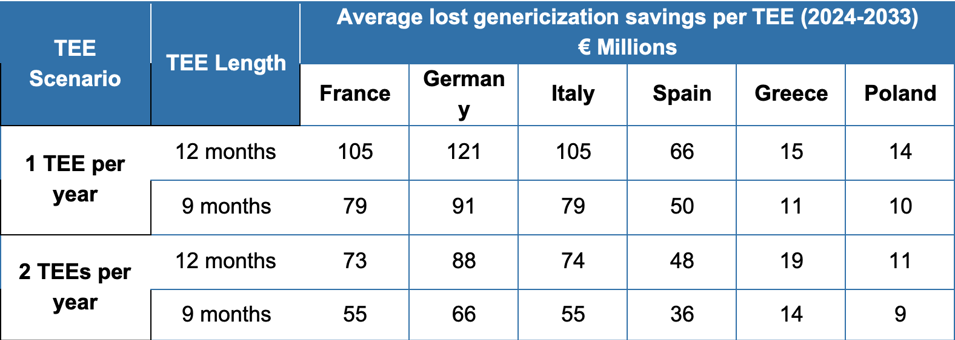
Even if research looks at only some of the benefits delivered by an antibiotic - and most deliver a range – the research shows overall that the costs incurred are outweighed by the net benefits.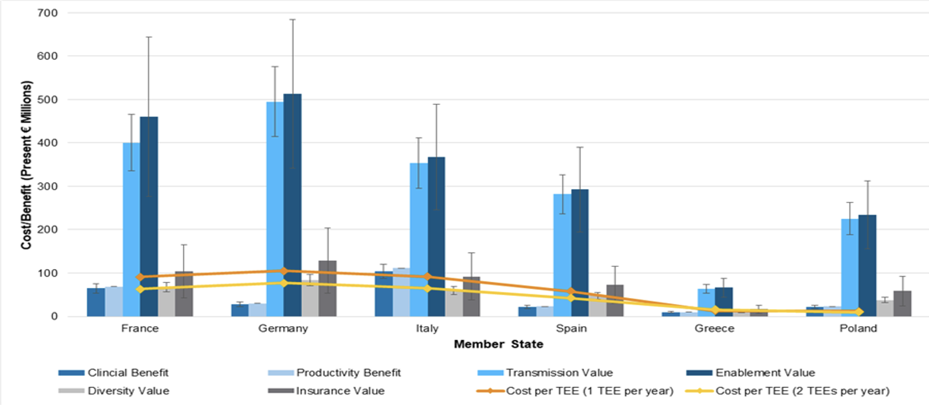
It is recognised that there is no one size fits all solution for global incentivisation of R&D for antibiotics, but the research suggests that not only is TEE is the most viable option for the EU, it would also deliver significant net benefits to all Member States. The costed, evidence-based proposal is the only existing option capable of incentivising R&D in a sustainable manner and within current EU frameworks, say authors. It facilitates participation from companies of any size and importantly it supports pro-stewardship, delinking payment from volume of use.
Antibiotics are the foundation of medical and surgical interventions. Authors say the cost-savings and benefits of TEE incentivising new antibiotics justify its use across other therapy areas.
Speaking about the report, EFPIA Director General, Nathalie Moll, said: “Finding a solution to the increasing danger of antibiotic resistance should be a priority for us all, and ensuring companies invest in R&D in this area is critical to achieving success.
Numerous ideas to stimulate new research have been proposed across the public and private sectors, and this research leaves little doubt that TEE is the best solution for Europe. Not only can TEE deliver new antibiotics it will bring significant economic benefits to each Member State.
The European Commission has committed to action on AMR and we believe TEE will give Europe the tools it needs to protect patients.”
Find out more on AMR here