What are Medicines Adaptive Pathways to Patients (MAPPs)?
MAPPs refer to flexible development and access pathways within the current regulatory framework that balance early patient access, public health and societal benefits. MAPPs start with the early authorisation of a product focused on a well-defined and targeted population with a clear safety and efficacy profile
The target population is adjusted as the evidence base expands. MAPPs may integrate adaptive clinical trial design, patient-centric benefit/risk assessments and continuous re-evaluation as new evidence becomes available. MAPPs, therefore, relate to the entire life cycle of a medicine from development, through licensing to patient access (reimbursement and healthcare delivery).

What drives MAPPs?
Scientific and technological progress today offers the capability of understanding diseases in a comprehensive manner and of identifying patients who would respond to a specific treatment. With the use of validated stratification tools, such as biomarkers, those patients that are most likely to respond can be preselected. This knowledge allows us to design more focused clinical trials and conduct them in an efficient manner, while obtaining the same or an even higher level of evidence.
Moreover, advances in information tools offer us the opportunity to collect real world data earlier on in the process and generate even more relevant evidence for other decision-makers, such as HTA bodies and payers.
Will MAPPs lower safety and efficacy standards?
No. MAPPs will employ well-established pharmacovigilance tools and will follow regulatory guidelines and studies set specifically for post authorisation safety and efficacy monitoring. Simultaneously, real life data will be collected supported by iterative and continuous benefit/risk evaluation cycles.
Will MAPPs require changes to legislation?
No. The applicability and feasibility of MAPPs is being explored within the existing legislative framework. Currently, available regulatory tools allow for investigating and developing MAPPs approaches and identifying areas that will require improvements.
Why are MAPPs are important and for whom?
Patients are expecting faster access to new medicines and the regulatory environment is lagging behind evolving science. Conventional R&D models are no longer financially viable and have become a major hurdle to efficient drug development, while general response rates to modern medicines are not satisfactory. A more flexible pathway would not only accelerate patient access to crucial therapies, but would also increase their probability of success as the therapies would be given to those patients most likely to respond.
However, a pre-requisite for the success of the implementation of MAPPs lies in the full and common understanding of its value, not just for industry, but across the entire innovation life cycle: for regulators; HTAs; payers; governments; clinicians; and, most importantly, patients.
How will MAPPs work?
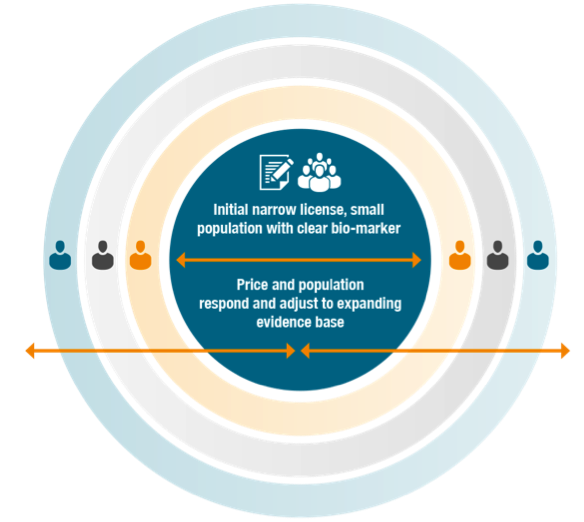
- MAPPs will focus initially on targeted, stratified medicines with clear biomarkers, well-defined populations, preferably an available diagnostic, and a high level of efficacy and safety
- MAPPs imply an initial marketing authorisation for a limited patient population upon demonstration of a positive benefit/risk balance
- It will generate confirmatory evidence from observational data sources while randomised clinical trials continue in support of the original or additional indications
- As more indications are validated, the population approved for the new medication will adapt to the evolving evidence base, as will the initial pricing and value assessment.
The evidence generation plan would be established in advance through joint Scientific Advice involving at least regulators and HTA/payers, or using other mechanisms for collaborative development or market access conversations. This process would include:
- Setting the data requirements for early regulatory approval based on initial evidence
- Iterative benefit-risk evaluations at pre-agreed milestones to:
– Confirm initial findings
– Consider expansion of the indicated population using evidence from real world and parallel clinical development
– Consider re-assessment of value, as longer-term effectiveness and outcomes are understood - Agreement on evidence package and methodologies for re-assessment
The evidence generation plan would be established in advance through joint Scientific Advice involving at least regulators and HTA/payers, or using other mechanisms for collaborative development or market access conversations. This process would include:
- Setting the data requirements for early regulatory approval based on initial evidence
- Iterative benefit-risk evaluations at pre-agreed milestones to:
– Confirm initial findings
– Consider expansion of the indicated population using evidence from real world and parallel clinical development
– Consider re-assessment of value, as longer-term effectiveness and outcomes are understood - Agreement on evidence package and methodologies for re-assessment
What are the challenges?
As MAPPs intends to bring therapies to patients earlier, it must rely on an infrastructure of validated stratification tools, capable of collecting data, identifying target patient groups and tracking the impact of specific medicines on their health. Current challenges include:
- The early, collaborative agreement by stakeholders (patients, regulators, HTA/payers, practitioners, industry) on the evidence package required for early regulatory approval and access for patients
- The IT infrastructure needed to provide the real world evidence base
Although already discussed in many public fora and supported by patients, regulators and the academic community, MAPPs has not been clearly defined yet; its feasibility within the current legislative framework and its viability for all stakeholders must be demonstrated and quantified. Three instruments are available for this purpose:
- The EMA Adaptive Pathways Pilot project – a regulator-led forum to simulate adaptive pathways, for example live assets.
- IMI multi-stakeholder coordination and stakeholder dialogue initiatives (IMI ADAPT-SMART)
What are the challenges?
As MAPPs intends to bring therapies to patients earlier, it must rely on an infrastructure of validated stratification tools, capable of collecting data, identifying target patient groups and tracking the impact of specific medicines on their health. Current challenges include:
- The early, collaborative agreement by stakeholders (patients, regulators, HTA/payers, practitioners, industry) on the evidence package required for early regulatory approval and access for patients
- The IT infrastructure needed to provide the real world evidence base