A sustainable environment for a healthy population (Guest Blog)
There is strong agreement between Bengt Mattson and Andreas Haener, chairs of the Interassociation Industry Pharmaceuticals in the Environment Task Force (IAI PiE TF) – industry has taken big steps over the past decade to combat the impact of pharmaceuticals in the environment. Bengt in particular has worked in the industry over the past 30 years, and led the IAI PiE TF since its set up over 15 years ago, ‘The industry has taken strides forward in minimising its emissions and driving environmental sustainability. Every stakeholder concerned must play its fair part and the industry has taken a leading role in this respect’.
Andreas, the environmental risk assessor for Roche, agrees wholeheartedly – ‘a collaborative approach is essential. Look at the IMI PREMIER Project, it brings together world-leading multi-disciplinary partners from the public and private sectors working to contribute to a sustainable future by proactively managing the environmental impact of medicines together. ‘
Introduction
The pharmaceutical industry acknowledges the impacts medicines and medicine’s development may have on the environment, that is why steps are being taken to ensure a healthier and environmentally more sustainable future. This can be accomplished by approaching an agile, innovative and evidence-based sustainability strategy to empower the industry to evolve in science, technology and society and to push sustainable, high-quality medicines across the entire value chain[1].
As we strive to move towards environmental sustainability, collaborating, coordinating, increasing conversations, listening, sharing, and learning with and from our partners is fundamental. Therefore, at EFPIA, we believe a cooperative approach with broader stakeholders to be the way that will allow us to expand our common knowledge and comprehension on how to proactively handle any potential risks imposed by the existence of Pharmaceuticals in the Environment (PiE). Consequently, EFPIA along with AESGP and Medicines for Europe have established the Eco-Pharmaco-Stewardship (EPS) framework with the strong focus on PiE and is executed across the industry and with broader stakeholders in the healthcare and environmental sector[2].
Our members are devoted to making a positive impact on the lives of patients whilst functioning in sustainable ways. As we have a responsibility towards the health of the population, we are moving forward in making a beneficial impact to the society by actively addressing concerns and raise awareness around risks concerning the impact on the environment while responding to patient needs and ensuring access to life-saving medicines.
Industry actions and the extended Environmental Risk Assessment concept
A risk-based approach is encouraged by the pharmaceutical industry to evaluate environmental challenges and thereby creating initiatives to endorse greater environmental responsibility across the industry and the society for water, chemicals, climate change and circular economy[3].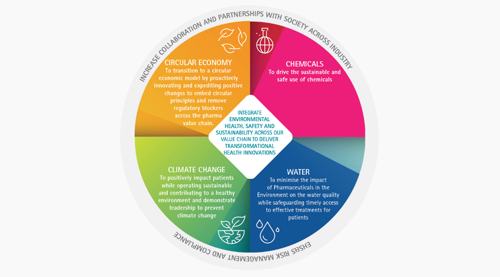 Although research driven pharmaceutical companies do not typically belong to high energy consuming companies, they are at the forefront of numerous ground-breaking initiatives to help reduce CO2 emissions. They are committed to contribute responsibly to progress in mitigating climate change and transitioning to circularity across the medicines life-cycle with regard to CO2 emission reduction targets, specifically addressing increased energy efficiency and lowered energy intensity across our value chains.
Although research driven pharmaceutical companies do not typically belong to high energy consuming companies, they are at the forefront of numerous ground-breaking initiatives to help reduce CO2 emissions. They are committed to contribute responsibly to progress in mitigating climate change and transitioning to circularity across the medicines life-cycle with regard to CO2 emission reduction targets, specifically addressing increased energy efficiency and lowered energy intensity across our value chains.
Furthermore, our members take responsibility for reducing environmental risks from both manufacturing emissions, through implementation of risk-based containment procedures in their manufacturing Effluent Management Programs and also extended producer responsibility (EPR) programs for waste pharmaceuticals and supporting the meds disposal campaign and other take-back schemes[4],[5]
In the coming weeks, as part of the pharmaceutical legislative review, the Commission will adopt legislation looking at strengthening the environmental risk assessment for medicines. Minimizing the impact of pharmaceuticals in the environment, the extended Environmental Risk Assessment (eERA) concept was proposed by the pharmaceutical industry to address the challenges and strengthen the Environmental Risk Assessment (ERA) process in the EU.
We believe that the ERA should be reviewed and, if necessary, updated throughout a product’s lifecycle to reflect the latest information on the medicine’s potential impact on the environment, while avoiding duplications of submissions for off-patent drugs. However, the focus should be on the active pharmaceutical ingredients (APIs) entering the environment and not on each single product, as a single API can be used in multiple products. Regulatory, academic and industry resources and associated environmental mitigation strategies should be prioritised on those APIs that pose a potential risk to the environment. This is why we have been working on the eERA concept.
The refinement of the existing ERA process for medicines would ensure that they remain up-to-date and relevant, capturing new reliable environmental toxicity data and measured environmental concentration. Newly available data from research can play an important role in the refined ERA process, extending beyond marketing authorisation. The ERA of a medicine is currently performed by companies either as part of a new marketing authorisation, line extension or when marketing authorisation for an existing product is expected to increase the environmental exposure of the API. The ERA must be performed to evaluate potential risks of medicines to the environment and ensure adequate precautions are taken where specific unacceptable risks are identified[6],[7],[8].
In summary the eERA aims to provide the following benefits[9],[10],[11],[12]:
- An API based ERA which better reflects the risks posed to environment from patient use
- Conduct robust and risk-based ERAs without compromising environmental protection or patient access to medicines
- Provision for the ability to automatically cross-reference ERA data in marketing authorisation applications
- Provide a mechanism for risk identification, refinement, and management during the MAA evaluation process
- Provide clarity on appropriate well-defined follow-up responsibilities for ERAs with no need for independent and duplicative risk identification and prioritisation processes under different legislations (e.g. Water Framework Directive)
- Updates to the ERA across the life cycle of the API in each medicinal product, with the latest environmental information
- A focus on risk that reduces the burden on regulators (i.e. oversight) and industry
- Reduction in the duplication of testing, delivering improved ERA consistency, proportionate use of testing resource, and bioethical benefits
- Increase the transparency of, and access to, ERA data
Furthermore, EFPIA members are committed to the science-based phase-in of methods to replace the use of animals for scientific purposes and the waiving of animal tests which are obsolete or redundant. Therefore, we believe that alternative and intelligent testing strategies such as the use of predictive in silico and in vitro tools can play an active role in reducing or avoiding unnecessary and particularly animal intensive testing required as part of the ERA. The prioritisation of testing of legacy APIs and development of intelligent testing methods has been, and continues to be, a significant research priority for the pharmaceutical industry and the European Commission through the Innovative Medicines Initiative (IMI).
ConclusionThe pharmaceutical industry is driven with motivation to advance human health and wellbeing in a sustainable way. Moreover, EFPIA welcomes and embraces the Commission’s emphasis on the Green Agenda and a more sustainable Europe, and is engaging constructively on the roll-out of their policy priorities. There are many risks associated in the whole life-cycle of a medicinal product that impact the environment negatively. Further understanding of these impacts and the interface between the society, health and the environment is the key to guarantee that the pharmaceutical industry can form and execute actions. Next to these initiatives, we as industry should create awareness to the users/patients and support them to act sustainable by properly disposing medicines. This should be a team effort as the overall goal is to create a sustainable environment for a healthy population.


