Changing Cancer Patients Lives – Reconciling Access, Affordability and Innovation
16.09.21
The economist Amyrta Sen noted that: “What moves us, reasonably enough, is not the realization that the world falls short of being completely just – which few of us expect – but that there are clearly remediable injustices around us which we want to eliminate.”[1] Cancer patients face a multitude of remediable injustices around us. Everyone involved in the research, funding and treatment of cancer can contribute to their elimination.
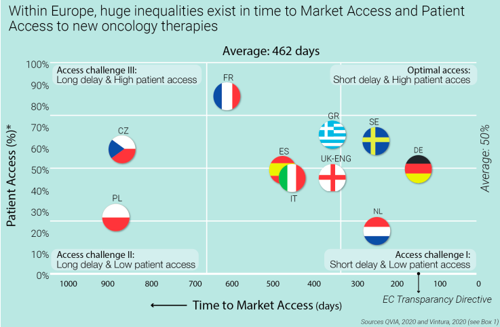
The recent report of Christel Jansen and Bas Amesz offered some interesting findings.[2] There are countries which provide speedy reimbursement, so time to patient access in Germany is one of the best while in other countries like France, it takes more than 600 days until a new medicine is reimbursed. But time to reimbursement is only one factor in patient access: In Germany, only half of the patients are treated with the new medicine after 12 months while in France it is more than 80 percent. Optimal patient access would be fast and broad access.

Access – everyone could do better
Jansen and Amesz also looked at the reasons for these differences: late submission, limited adherence to timelines, multiple layers of decision-making, evidence gaps, misalignment on value and price etc. In short: Many causes undermining patient access, and many stakeholders involved.
Besides time to patient access, another important factor is affordability. Countries, even regions, differ in terms of income. It is generally accepted that price levels in Latvia are different from the ones in Switzerland. Differential pricing, i.e. pricing according to ability to pay, has been discussed for a long time in many reports from multilateral organizations and the European Institutions.[3]
Policies impact patient access
However, differential pricing is not possible if external reference pricing is in place. Countries referencing each other may undermine this effort if reference is made to a lower economy. As one of the papers notes: “Significant political will to agree on principles and mechanisms for differential pricing, including willingness for transparency around differentially set prices, would be needed.”[4]
Whether this approach – centrally regulated differential pricing with transparent prices – is the right one will be viewed differently by different stakeholders. Nevertheless, there is consensus that external price referencing undermines differential pricing.
Innovation doesn’t stop
Access and affordability are key for patient benefit. Innovation is meaningless without access. But it is also true that access and affordability are empty concepts if there is no innovation. Today, one out of two patients with metastatic skin cancer can expect to be alive 5 years after diagnosis. Then years ago it was ten times less.[5] Recent data on lung cancer in the United States showed that mortality decreased by 3.2% annually between 2006 and 2013, and more quickly, by 6.3% annually from 2013 to 2016 which is a result of prevention and new treatments.[6]
Another example of continuous innovation comes from Hepatitis C: Initially, breakthrough treatments introduced in 2014 led to triggered a huge public debate and in some countries restrictive policy responses to contain costs.[7] But the story continued: A few years later, prices had dropped by more than 50% with incoming competition – not from generics but from other innovators. Not less than seven other treatments came to market. The advent of competition has delivered significant improvements in the quality of life for patients: the numbers of pills per day were reduced from 6-12 to 1 per day. And value for society: the treatment duration went down from 24-48 weeks to 8-12 weeks.
Better sooner than later …
This example illustrates the inherent tension between access, affordability, and innovation. Patient benefit is every stakeholder’s responsibility. And as this example shows, industry should better prepare for making disruptive innovation accessible.
Personalized Oncology – as presented in a recent EFPIA supported report of the London School of Economics – will be the future of cancer care but also faces several challenges as genomic testing needs to be better integrated in health systems.[8] We must have a dialogue with all stakeholders to ensure that these treatments become accessible and affordable. Similar challenges will occur from combination treatments or tumor-agnostic treatments and novel endpoints, all topics the EFPIA Oncology Platform is working on now.
Let’s be pragmatic
There are also some good practices from the past where industry and governments have found pragmatic solutions to reconcile access, affordability, and innovation.[9] In the field of multi-indication medicines, molecules which can treat more than 30 cancer types, Germany for example has introduced a “bundling” mechanism to accelerate negotiations. Belgium and the Netherlands have developed so-called multi-year, multi-indication contracts which reduced time to access and were useful in managing budget impact and uncertainty. In the case of Belgium, time to patient access has been reduced from 400 to 30 days.
We must not be blinded by the fact that reconciling access, affordability, and innovation is impossible. Alternatively, we should explore pragmatic approaches to eliminate “clearly remediable injustices”. Collaboration in this context means sometimes difficult conversations but with a meaningful outcome: restoring the hope of cancer patients.
[1] Sen A (2009), The idea of justice; Allan Lane & Harvard University Press
[2] Jansen C, Amesz B (2020), Every Day Counts; Vintura; https://www.efpia.eu/publications/downloads/efpia/every-day-counts-improving-time-to-patient-access-to-innovative-oncology-therapies-in-europe/
[3] E.g. OECD (2018), Pharmaceutical Innovation and Access to Medicines, OECD Health Policy Studies, OECD
Publishing, Paris; GOEG-Sogeti (2015), Study on enhanced cross-country coordination in the area of pharmaceutical product pricing; European Commission; Vogler S, Paris V, Panteli D (2018), Ensuring access to medicines: How to redesign pricing, reimbursement and procurement. Policy Brief 30; European Observatory on Health Systems and Policies
[4] Vogler S, Paris V, Panteli D (2018), Ensuring access to medicines: How to redesign pricing, reimbursement and procurement. Policy Brief 30; European Observatory on Health Systems and Policies
[5] ESMO (2019), One in Two Patients with Metastatic Melanoma Alive after Five Years with Combination
Immunotherapy; https://www.esmo.org/newsroom/press-office/esmo-congress-melanoma-immunotherapy-checkmate067-larkin
[6] Howlader N et al. (2020), The Effect of Advances in Lung-Cancer Treatment on Population Mortality; NEJM 383:640-9
[7] Roediger A et al. (2019), Competition between on-patent medicines in Europe; Health Policy 123;7:652-660
[8] Gill J, Fontrier AM, Miracolo A, Kanavos P (2020), Access to Personalized Oncology in Europe; London School of Economics; https://www.efpia.eu/media/580518/access-to-personalised-oncology-in-europe.pdf
[9] Lawlor R et al. (2021), Accelerating patient access to oncology medicines with multiple indications in Europe; J Market Access & Health Policy
