Equitable access to innovative COVID-19 vaccines in Africa should focus on healthcare system absorption capacities & vaccine confidence
So how did we get to this situation?
Production and deliveries of COVID-19 vaccines
The Africa CDC announced it will ask the world to pause COVID-19 vaccine donations. How did we get to this situation?
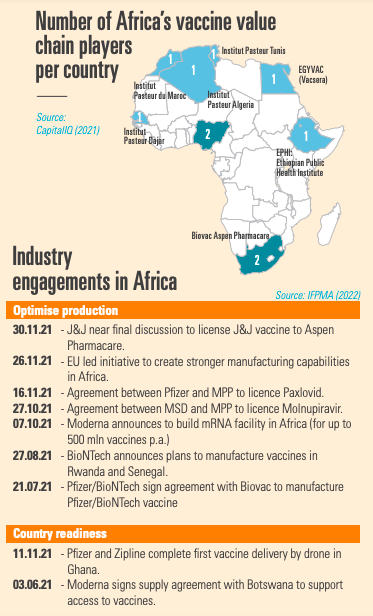
Since the beginning of the pandemic, industry has committed to increase equitable access to innovation and to safe, effective, and quality-assured essential medicines and vaccines globally.
COVID-19 vaccine production (also for mRNA vaccines) is very complex[3], takes between 2-4 months, involves 50,000 complex process steps, and requires 280 ingredients for some vaccines. To ensure the highest level of quality, 70% of the time to produce a vaccine is spent on quality control.[4] To this end, 357 collaborations, of which 89% include technology transfers, have been set up.[5] An important and positive learning from the pandemic has been the effectiveness of these voluntary collaborations.
A flexible voluntary system has allowed different players from different parts of the world to come together to scale up and address healthcare challenges. These players have been carefully selected from hundreds of screened sites based on quality standards, compliance safety track records, technical capabilities, capacity availabilities, certifications, trained workforce and project management capabilities. This flexibility has been critical to address the pandemic and will be critical to meet any future healthcare challenges.
Healthcare system absorption capacity and vaccine acceptance
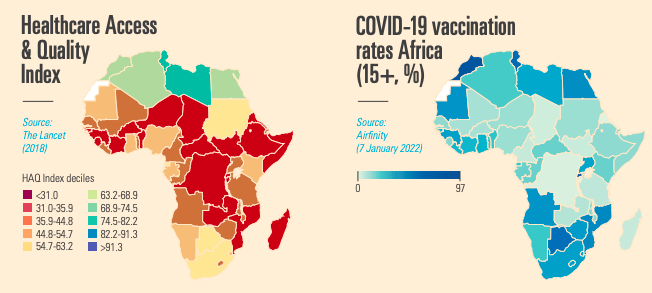
But while manufacturing and technology transfers are definitely part of the big picture – it is essential to look at healthcare system absorption capacities and low rates of vaccine acceptance.
These are two key challenges today, identified by the WHO to vaccine implementation (alongside coordination, number of healthcare workers, delivery funding, complex vaccine handling, and stringent storage requirements).[6] There is a strong correlation between healthcare system capacities and vaccination rates.
So, to the above question “How did we get to this situation?”, John Nkengasong, director of the Africa Centres for Disease Control, said: “the primary challenge for vaccinating the continent is no longer supply shortages but logistics challenges and vaccine hesitancy“— leading the agency and the African Vaccine Acquisition Trust to ask for the delay in vaccine deliveries.
Conclusions
With the ultimate goal of getting as many citizens vaccinated as possible against COVID-19, strengthening the varied healthcare system capacities across Africa is critical in the immediate future. Equally important is sharing clear information to increase vaccine confidence. These are key policies needed to achieve vaccine equity globally.
At the same time, it is important to start the dialogue – with all relevant stakeholders around the table – in the context of the EU-AU relationship to find ways to build up manufacturing capacity for vaccines and treatments in Africa, to support African and EU resilience in the long-run, including for future pandemics, facilitated by international trade and investment. This topic we will address in more detail in the next blog.
[1] Airfinity (2022)
[2] Our World in Data “Share of people vaccinated against COVID-19, February 17, 2022” [accessed 18.2.2022]
[3] PIE (2020)
[4] Vaccines Europe (2021)
[5] Airfinity (2022)
[6] WHO (2021)