Everyday counts: 9 reasons why patients wait longer to get access to new cancer medicines
24.01.20
As outlined by the newly published COMPARATOR REPORT ON CANCER IN EUROPE 2019[1] by the Swedish Institute for Health Economics, outcomes for cancer patients are improving. Cancer mortality is slowing down. Survival rates are improving, giving many people the chance to live longer with better quality of life. These improved cancer outcomes are driven by several factors, including effective prevention, screening programmes and timely diagnosis. In addition, advances in health technologies have paved the way for further improvements in cancer survival.
Nevertheless, it would be too early to be complacent. Still, the number of people getting cancer has increased significantly since 1995 – due to population aging, lifestyle changes, and improved diagnosis. In addition, as discussed in our previous blog “Everyday Counts: Time to Patient Access to Innovative Oncology Therapies in Europe”, not every patient in Europe is able to benefit from these advances. Large inequality exists across Europe on patient access to new cancer treatments. As illustrated in the Patient W.A.I.T indicator[2] survey conducted by the European Federation of Pharmaceutical Industries and Associations (EFPIA) in 2018, the average time to reimbursement for cancer treatments across EU29 countries continues to take as long as 445 days, ranging from 119 days in Germany to over 900 days in Serbia.
That’s why the EFPIA Oncology Platform has decided to support an initiative named Time to Patient Access, which brings together diverse stakeholders across Europe to jointly identify factors which cause access delays, and co-create solutions that could accelerate time to patient access.
Over the past 6 months, stakeholders from patient organisations, regulators, policy makers, health technology assessment (HTA) bodies, payers, leading health economists, and the industry have been engaging in open, transparent dialogues on this topic. Literature reviews and extensive telephone interviews with over 40 access stakeholders across European countries have also been conducted to enrich the research and analysis. This broad collaboration has now led to a significant milestone: the identification of nine key factors which cause delays to patient access.
These nine factors carefully examine the existing healthcare operating system across Europe, as well as some of the existing rules and regulations which result in unintended consequences. As outlined in the table below, these nine factors focus on facts, systems and policies from the perspectives of Process, Value Assessment Criteria, and Health System Readiness, thus removing the traditional blaming and shaming, and enabling constructive assessment and problem-solving.
9 factors delaying patient access to innovative oncology therapies in Europe
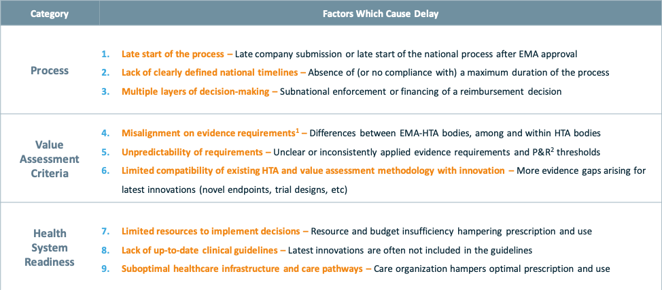

Source: Stakeholder Sounding Board of the project “Time to Patient Access” 1 Population, comparator, endpoints, trial design or analysis; 2 Pricing and reimbursement.
Process:
1) Late start of the process: Late start of the reimbursement process has been recognized as one of the major delaying factors. This includes the late submission of reimbursement dossiers by pharmaceutical companies due to external reference pricing (ERP) and its negative impact on prices. As pointed out by Kanavos et al (2019)[3], ERP can lead to launch delays, launch sequencing or no launch. Late start of the process can also include late start of the national reimbursement processes, as some countries want to wait for the formal EMA decision and/or reimbursement decisions in other countries before they start their own reimbursement processes[4].
Process:
1) Late start of the process: Late start of the reimbursement process has been recognized as one of the major delaying factors. This includes the late submission of reimbursement dossiers by pharmaceutical companies due to external reference pricing (ERP) and its negative impact on prices. As pointed out by Kanavos et al (2019)[3], ERP can lead to launch delays, launch sequencing or no launch. Late start of the process can also include late start of the national reimbursement processes, as some countries want to wait for the formal EMA decision and/or reimbursement decisions in other countries before they start their own reimbursement processes[4].
2) Lack of clearly defined national timelines: Although the EU Transparency Directive (Directive 89/105/EEC) has set 180 days as the maximum timeline for member states to make pricing and reimbursement decisions, in most countries this timeline is not adhered to, due to reasons such as capacity, lack of clearly defined national timelines, etc
3) Multiple layers of decision-making processes: in some countries, reimbursement decisions need to be made at all levels from national level to regional level and to then local hospital level, thus prolonging the time before patients can access treatments.
Value assessment criteria:
1) Misalignment on evidence requirement: Misalignment on evidence is probably one of the most prominent and complex delaying factors. Misalignment takes place not only between industry, regulators, and HTA bodies, but also occurs between regulators and HTA bodies, as well as among different HTA bodies. Misalignment can be found in all assessment criteria ranging from patient population, comparators, trial design, end points, to statistical analysis.
2) Unpredictability of requirements: in addition to misalignment between different HTA bodies, even within the same HTA body, the application of the evidence requirements and thus the assessment criteria is not always consistent. The absence of clearly defined (or inconsistent application of) evidence requirements and value judgement criteria often prolongs assessment process and decision-making.
3) Limited compatibility of existing HTA and value assessment methodology with innovation: health technologies are advancing at an unprecedented pace, with new targeted therapies, cell and gene therapies, and tumor-agnostic therapies. In addition, cancers are more commonly being treated with complex combinations of new and/or existing medicines, with or without biomarker testing. On the other hand, HTA criteria and value assessment methodologies remain largely unchanged in the face of this new level of innovation. In situations such as combination therapies, no HTA methodology or value assessment process currently provides clear and consistent value assessment and distribution. This lack of compatibility between existing assessment methodologies and the latest innovations in oncology is resulting in delayed access to new therapies that have the potential to significantly extend life.
1) Misalignment on evidence requirement: Misalignment on evidence is probably one of the most prominent and complex delaying factors. Misalignment takes place not only between industry, regulators, and HTA bodies, but also occurs between regulators and HTA bodies, as well as among different HTA bodies. Misalignment can be found in all assessment criteria ranging from patient population, comparators, trial design, end points, to statistical analysis.
2) Unpredictability of requirements: in addition to misalignment between different HTA bodies, even within the same HTA body, the application of the evidence requirements and thus the assessment criteria is not always consistent. The absence of clearly defined (or inconsistent application of) evidence requirements and value judgement criteria often prolongs assessment process and decision-making.
3) Limited compatibility of existing HTA and value assessment methodology with innovation: health technologies are advancing at an unprecedented pace, with new targeted therapies, cell and gene therapies, and tumor-agnostic therapies. In addition, cancers are more commonly being treated with complex combinations of new and/or existing medicines, with or without biomarker testing. On the other hand, HTA criteria and value assessment methodologies remain largely unchanged in the face of this new level of innovation. In situations such as combination therapies, no HTA methodology or value assessment process currently provides clear and consistent value assessment and distribution. This lack of compatibility between existing assessment methodologies and the latest innovations in oncology is resulting in delayed access to new therapies that have the potential to significantly extend life.
Health system readiness:
1) Limited resources to implement decisions: delays to patient access not only take place prior to the public reimbursement of oncology therapies, but also occur after reimbursement decisions. One of the reasons is the resources and budget constraint at regional or local hospital level which limit the use and uptake of the treatments in clinical practices.
2) Lack of up-to-date clinical guidelines: many clinical guidelines are not regularly updated to include the latest innovative therapies. As a result, despite innovative therapies are publicly reimbursed, their clinical use will be limited as healthcare professionals often follow the clinical guidelines.
3) Suboptimal healthcare infrastructure and care pathways: In addition to resources and clinical guidelines, other factors that delay or hamper clinical use of innovative therapies include the lack of referral, limited access to diagnosis and/or biomarker testing, training to healthcare professionals, etc.
1) Limited resources to implement decisions: delays to patient access not only take place prior to the public reimbursement of oncology therapies, but also occur after reimbursement decisions. One of the reasons is the resources and budget constraint at regional or local hospital level which limit the use and uptake of the treatments in clinical practices.
2) Lack of up-to-date clinical guidelines: many clinical guidelines are not regularly updated to include the latest innovative therapies. As a result, despite innovative therapies are publicly reimbursed, their clinical use will be limited as healthcare professionals often follow the clinical guidelines.
3) Suboptimal healthcare infrastructure and care pathways: In addition to resources and clinical guidelines, other factors that delay or hamper clinical use of innovative therapies include the lack of referral, limited access to diagnosis and/or biomarker testing, training to healthcare professionals, etc.
While it has been a major task by this stakeholder group to identify and agree upon the key reasons for delay, the real challenge is now to co-create and execute tangible solutions. As of today, six potential solution areas have been crafted, and certainly more needs to be done to refine and pressure-test these solution areas. We therefore invite stakeholders who are interested to be part of these solutions to join us on 29th Jan 2020 in Brussels, at our final Stakeholder Sounding Board meeting. For more information about the event, please email: cJansen@vintura.com. All insights and contributions are welcomed!
EVERYDAY COUNTS!
[1] Hofmarcher, T., Brådvik, G., Svedman, C., Lindgren, P., Jönsson, B., Wilking, N. Comparator Report on Cancer in Europe 2019 – Disease Burden, Costs and Access to Medicines. IHE Report 2019:7. IHE: Lund, Sweden
[2] EFPIA Patient W.A.I.T. Indicator 2018 survey. “Patients W.A.I.T.” stands for Patients Waiting to Access Innovative Therapies. The INDICATOR provides a benchmark of the rate of availability and waiting times in European countries
[3] Panteli, D. et al. Pharmaceutical regulation in 15 European countries: Review. Health Systems in Transition, 2016.
Kanavos, P. et al. Does external reference pricing deliver what it promises? Evidence on its impact at national level. Eur J Health Econ, 2019
[4] Greece: article 22 of Law 4633/2019: medicines with patent protection are subject to HTA assessment in Greece only if they are reimbursed in 5 other countries with HTA assessment process from the following list: Austria, Belgium, France, Germany, Denmark, Spain, Netherlands, Italy, Portugal, Sweden and Finland.

