Innovation:
Putting power tools to work
Innovation:
Putting power tools to work
Future health systems must embrace innovations that generate value.
The innovation imperative
The COVID-19 pandemic has underscored the critical importance of research and innovation for public health, economies and society as a whole.
It has triggered an unprecedented mobilisation involving public research agencies, academia, private foundations and charities, and industry. These efforts have delivered astonishing progress in record time, both in terms of understanding this new disease, devising public health strategies, providing treatments and delivering an array of highly effective vaccines (See Resilience).
The European research-based pharmaceutical industry sector was well placed to play a leading role in the global response to COVID-19 because of its long-standing commitment and investment in research and innovation in Europe. In particular, we have:
- led the search for vaccines, diagnostics and treatments to find a permanent route out of COVID-19: currently worldwide more than 288 vaccine candidates are in development – 104 of these are in clinical trials. Four vaccines have been approved by the European Medicines Agency.
- ensured the supply of medicines, linking manufacturers and the European Medicines Agency in order to anticipate and proactively address any potential disruptions.
- supported governments in strengthening their health systems by ramping up the production of successful vaccines, and to ensuring that approved treatments and vaccines are available and affordable.
We believe in the power of innovation to help address the challenges facing health systems – sustainably improving and extending lives while increasing value for money, and tackling emergency threats. Innovation in this context means not only medicines and vaccines, but diagnostics, imaging, medical devices and surgical techniques, together with integrated models for health service design, delivery, management and financing.
To help POWER up health systems, Europe must put innovation to work holistically across all parts of the health system, where it generates value for patients and the system as a whole. Together we need to ensure that European health systems have the power tools they need.
Incentivising innovation that generates value
During the COVID-19 pandemic, the research-based pharmaceutical industry has joined forces with other sectors to develop diagnostics, treatments and vaccines with an unprecedented speed and success. Regulators and policymakers have streamlined regulatory processes and employed emergency measures to facilitate the search for potential treatments and vaccines.
As well as reflecting on how we can use these learnings to optimise clinical trials (See Resilience), we should use this momentum to harness innovation to POWER up healthcare systems. This can only be done by co-ordinated EU and national policies and ecosystems that embrace innovations that bring value to patients and health systems.
Value and affordability: taking a holistic approach
The current fragmentation and siloed nature of budgets within and across health and social systems means that the value and affordability of pharmaceuticals and other health innovations tends to be considered only within the limited context of a specific budget. As a result, the value that innovative treatments can provide across healthcare budgets cannot be properly assessed, leveraged or incentivised to allow patients, health systems and society to reap the full benefit.
In addition to bringing the promise of longer and healthier lives for patients, many innovations can help to reduce costs in other parts of the health or social care systems, for example by reducing the need for specialist medical or surgical care, acute hospitalisations, palliative care or long-term nursing care.
Discussions about ‘affordability’ should therefore not be focussed on pharmaceutical spending in isolation, but according to the value that innovation generates for patients and health systems throughout the continuum of care and across all components of the health, social care and welfare system. To this end, regulation and HTA will need to embrace patient perspectives (including patient-reported outcomes) and robust real-world data.
To illustrate the importance of this holistic approach:
- According to the OECD, on average, pharmaceuticals (both retail and hospital) account for around one fifth of total health systems expenditure across the EU. Pharmaceuticals spending varies considerably between countries, both as a percentage of health spending and GDP. Pharmaceutical spending was relatively stable, rising by an average of 1.4% per year between 2013 and 2018, after falling by 1.2% per year between 2008 and 2013 owing to cost-containment policies. By contrast, average spending on long-term care related to ill health rose three-fold more quickly, at 4.3–4.4% per year, throughout 2008–2018. Health-related long-term care already accounts for a quarter of health spending in the Netherlands, Sweden and Denmark, compared with an EU average of 12%. The OECD projects that expenditure on long-term care as a percentage of GDP could double by 2060. Innovative treatments and services that reduce demands for long-term care therefore have enormous potential value to society.
- Innovative treatments may also allow patients to return to work, thereby paying taxes and contributing to economic productivity and cutting costs of welfare support. One recent study estimated that comprehensive investment in care for 11 cancers in high-income countries – representing a 6% increase in costs – would provide a 3.7-fold return on investment by 2030 owing to productivity gains in the economy. The highest return was estimated from imaging (129-fold) and treatment (17-fold).
- The panel below provides further examples of how specific types of innovative medicines can bring value across sectors.
Affordability should also be interpreted in light of the estimate that health systems waste approximately as much money (up to 20% of resources) as they spend on all pharmaceuticals combined, and yet often have limited ability to redirect spending to improve efficiency.
Value gains from innovation:
Examples of potential value gains from specific innovations include:
Novel payment models
Our industry believes that, when used appropriately, novel pricing and payment models can accelerate patient access, allowing payers to manage clinical uncertainty, budget impact and sustainability of the healthcare system, whilst providing sufficient incentives for innovation.
In contrast to conventional pricing models (such as simple discounts, rebates, price-volume agreements or caps), novel pricing approaches relate the price of a product to its value to patients and health systems. Novel payment approaches condition payment according to the value of the product (in terms of observable outcomes), spread payment over time, or decouple payment from the number of patients treated (see Panel).
Based on the experience of using these models today, there is already evidence to show how they can accelerate and broaden patient access, and contribute to improving the sustainability of healthcare systems.
Key examples of novel pricing and payment models
However, the lack of appropriate data infrastructure, legal barriers and an unwillingness to adapt current systems often prevent the use of these models.
The EFPIA report Novel pricing and payment models: new solutions to improve patient access proposed the following principles to guide the use of novel payment models and to help overcome these barriers:
- Access Principle: Novel pricing and payment models should facilitate broad and timely patient access whilst balancing the sustainability of the healthcare system and incentives for innovation.
- Value Principle: A high quality, methodologically robust and mutually agreed value-based framework is the foundation for novel pricing and payment models.
- Collaboration Principle: Payers and companies should work together to anticipate where these models are needed and ensure they are fit for purpose.
- Transparency Principle: The existence of novel agreements should be transparent and disclosed, and information collected should be shared according to specifically defined principles, including mutual agreement and patient confidentiality. The industry is committed to working constructively with the European Commission, national policymakers and other stakeholders to advance the much-needed cross-stakeholder dialogue on transparency. Open collaboration and a shared commitment among payers, policymakers and the industry are required, together with the active engagement of healthcare professionals and patient organisations.
- Infrastructure Principle: Stakeholders should work together to ensure the required data infrastructure is fit for purpose and appropriate legal frameworks are in place.
Blocking innovation impedes POWER
Patients WAIT survey
Overall, in 2019, the average delay between market authorisation and patient access varied by more than six-fold across Europe. Patients in Northern/Western Europe could access new products 100–350 days after market authorisation, compared with 600-850 days in Southern/Eastern Europe. For some products, variations within some countries were even larger than variations between countries.
To exemplify the variation, patients in Estonia and Latvia on average had to wait over 900 days until novel cancer treatments were publicly reimbursed. That is 28 times longer than patients in Germany. Patients in France had to wait 560 days, 15 times longer than those in Germany. In this regard, Germany has demonstrated how any similarly high-income country can accelerate access to innovation that adds value. We acknowledge that countries in other parts of Europe face particular resource pressures.
For orphan medicines, the average delay between market authorisation and patient access for Orphan drugs varied from 3.7 months to 3.2 years. Moreover, 15% of countries had no access to any of the orphan medicines approved between 2015 and 2018.
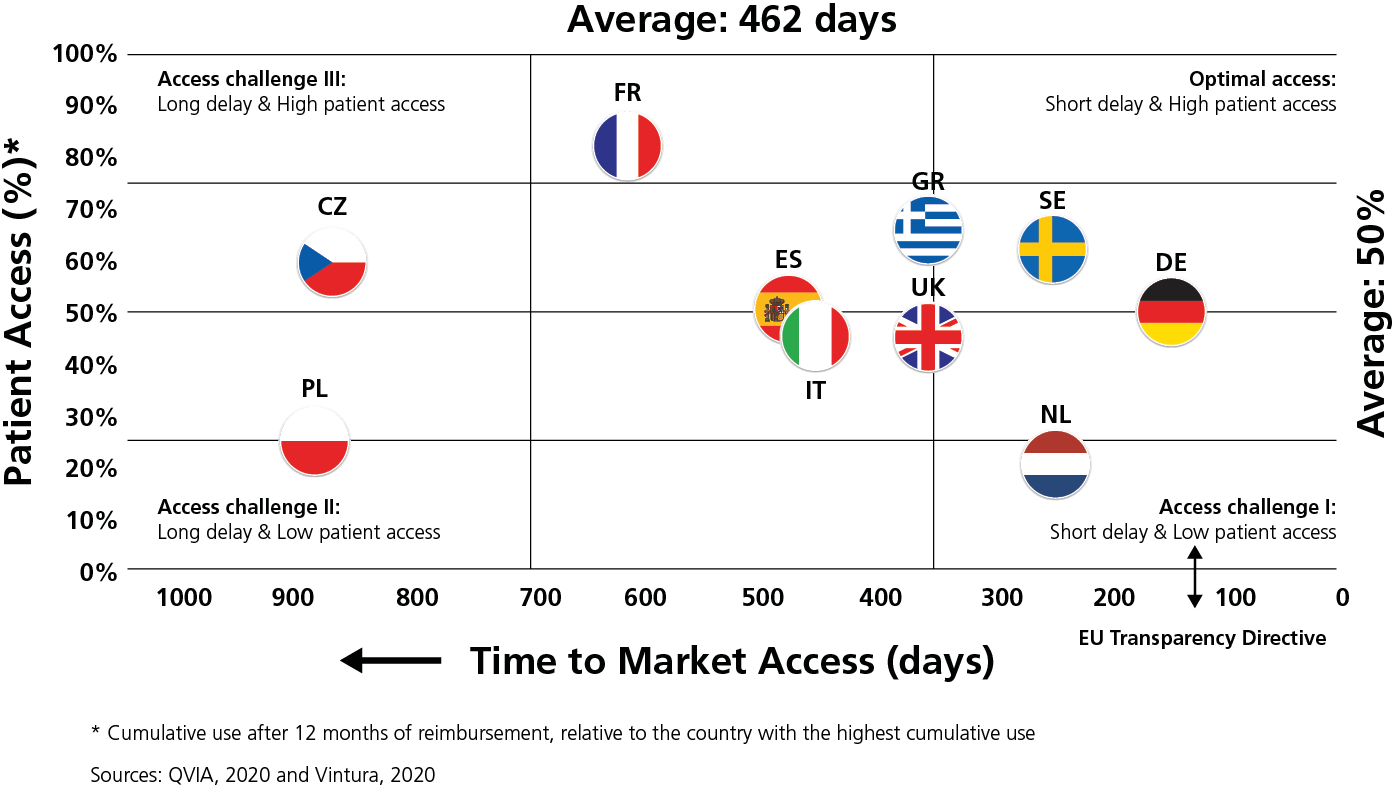
“Within Europe, huge inequalities exist in time to Market Access and Patient Access to new oncology therapies.”
 Everyone involved in healthcare – from patients to service providers, researchers to clinicians, pharmaceutical companies to payers – wants patients to be able access new treatment options equitably across Europe.
Everyone involved in healthcare – from patients to service providers, researchers to clinicians, pharmaceutical companies to payers – wants patients to be able access new treatment options equitably across Europe.EFPIA has identified 10 interrelated factors that explain unavailability and delays. These are rooted in the medicines access systems and processes in the EU Member States and the corresponding impact on commercial decision-making. They range from a slow regulatory process to late initiation of market access assessment, to duplicative evidence requirements, to reimbursement delays, and local formulary decisions, together with readiness of the health system to deliver innovative therapies (e.g. infrastructure, workforce enablement and suitable care pathways and guidelines). As the root causes are multifactorial, they can only be solved by different stakeholders working together.
The industry is a key partner to governments, regulatory authorities, and patients in ensuring that we all have an effective innovation ecosystem that drives new treatments to patients as fast as possible. We therefore welcome collaborative EU-level dialogue to identify the root causes of access disparities, delays and barriers and to design solutions.
Access solutions will also require a close collaboration of national healthcare systems (including HTA and pricing and reimbursement bodies) on issues such as pan-European evidence generation, meaningful joint clinical assessments, and novel pricing and payment models.
Together with our partners in the EU Health Coalition, we call for a permanent, high-level, multi-stakeholder Forum for better access to innovation. This would provide a space where all stakeholders – Member States, national and regional authorities, patients, civil society, healthcare professionals, policymakers, and industry can discuss the factors driving and impeding patients’ access to all types of health innovation.
The EU should be a global hub for research and innovation in health. We need to build a long-term, collaborative ecosystem that supports and incentivises research and development, including through interdisciplinary networks, cross-border initiatives, health data infrastructures, public-private and public-public partnerships, as called for by the EU Health Coalition.
To this end, EFPIA is partnering with other industry associations in the Innovative Health Initiative (IHI), a new proposed Joint Undertaking (public-private partnership) co-funded by the European Union under Horizon Europe. The IHI aims to enable the cross-sectoral integration of technologies, know-how, products, services and workflows for people-centred healthcare. Its ambition is to support the delivery of timely and well-substantiated prevention, diagnosis and treatment. The partnership aims to help keep EU citizens in good health, decrease disease burden for patients, care givers and health care professionals. It will also contribute to the sustainability and resilience of health care systems, to the competitiveness of health industries and to the EU technological strategic autonomy.
To be fit for purpose, such partnerships need to include:
- Transparent, open, and efficient processes that engage all relevant stakeholders in defining priorities and developing calls
- A solid intellectual property framework
- Suitable conditions that allow industry to contribute valuable assets to the Partnership that would otherwise not be accessible to the research community
- Possibility to enable global collaboration by bringing expertise and assets into EU projects from third countries and research hubs abroad.
Industry is already working on numerous public-private partnerships via the Innovative Medicines Initiative, many of which are featured throughout this paper.
Industry: partnering for a thriving innovation ecosystem
References
De la Maisonneuve C, Martins. Public spending on health and long-term care: a new set of projections. OECD, 2013 https://www.oecd.org/economy/growth/Health%20FINAL.pdf
EU Health Coalition. A shared vision for the future of health in Europe: lessons learnt from the COVID-19 pandemic. 2020. Available at https://www.euhealthcoalition.eu/recommendations/
EFPIA. Addressing healthcare challenges. Novel pricing and payment models: new solutions to improve patient access. 2020. Available at https://efpia.eu/media/554543/novel-pricing-and-payment-models-new-solutions-to-improve-patient-access-300630.pdf
Innovative Health Initiative. Strategic research and innovation agenda, 2021.
Available at https://www.euhealthppp.org/strategic-agenda
IQVIA. EFPIA Patients wait indicator survey. 2020.
Available at https://www.efpia.eu/media/554526/patients-wait-indicator-2019.pdf
EFPIA. The root cause of unavailability and delay to innovative medicines: reducing the time before patients have access to innovative medicines. 2021. Available at https://www.efpia.eu/media/602653/root-cause-unavailability-delays-cra-report-may-2021-final.pdf
EFPIA. Strengthening health systems through smart spending. 2020 https://www.efpia.eu/news-events/the-efpia-view/statements-press-releases/strengthening-health-systems-through-smart-spending/
EFPIA. Healthier future. The case for outcomes-based sustainable healthcare. 2020.
Available at https://www.efpia.eu/media/412313/the-case-for-outcomes-document-17102018.pdf
IQVIA. EFPIA pipeline review 2021 update. https://www.efpia.eu/media/602563/iqvia_efpia_pipeline-review_final.pdf
OECD/European Union. Health at a Glance: Europe 2020: State of Health in the EU Cycle. OECD, 2020. Available at https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-europe-2020_82129230-en?_ga=2.52510440.1357533625.1626362665-1150508910.1626362665
OECD. Tackling wasteful spending on health. 2017. Available at https://www.oecd.org/health/tackling-wasteful-spending-on-health-9789264266414-en.htm
OECD. Addressing challenges in access to oncology medicines. OECD, 2020. Available at https://www.oecd.org/health/health-systems/Addressing-Challenges-in-Access-to-Oncology-Medicines-Analytical-Report.pdf
OECD. Pharmaceutical spending (indicator). 2021 https://data.oecd.org/healthres/pharmaceutical-spending.htm
Vintura. Every day counts – improving the time to patient access to innovative oncology therapies in Europe. 2020 https://www.vintura.com/wp-content/uploads/2020/08/White-paper-every-day-counts-improving-time-to-patient-access-to-innovative-oncology-therapies-in-europe_from-EFPIA_and_Vintura.pdf
Ward ZJ, et al. Global costs, health benefits, and economic benefits of scaling up treatment and imaging modalities for survival of 11 cancers: a simulation-based analysis. Lancet Oncol 2021;22:341–50
World Health Organization. COVID-19 vaccine tracker and landscape. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (13 July 2021)